Final PDF Poster Submission must be received by 05 September 2025 for consideration.
Poster SubmissionTimeline
-
Final PDF Poster Submission Date
05 September 2025
Deadline for final printable PDF submission
Each abstract must include the following information to be considered:
- Presentation Title
- Presenter's Name And Contact Details
-
Presenter's Biography
(Approx. 100 words) -
Additional Speakers
(If applicable) - Key Objectives of Topic
-
2-3 Paragraph Abstract, Summarizing the Topic
(Max 200 words)
General Information
Abstract submitters may submit up to two entries for consideration.
All presentations must be free of commercial intent. Incomplete proposals will not be considered.
Call for Posters/Case Studies
Types of Submissions
Poster
Accepted posters will:
- Will be displayed in the exhibit hall and may also be included in the guided poster walk
Get the Attention you deserve!
Maximize your visibility by contributing a podium or poster presentation at PDA Universe of Pre-Filled Syringes and Injection Devices Conference 2025. Leverage this unique opportunity to highlight your work at the event. Posters will be available online post-event.
Topics we are looking for:
- 1. Managing the Regulatory Landscape
- New Guidance Documents
(e.g., EU GMP Annex 1) - Medical Devices, Combination Products, and Sustainability Regulations
- ISO/American Society for Testing and Materials/Pharmacopeial Standards Development and Harmonization
- Continuous Improvement and Regulatory Requirements including Global Inspections
- Drug Safety/Anti-Counterfeiting
- New Guidance Documents
- 2. Development and Trends
- Formulation/Container/Device/Functionality/Usability including Large Volume Injections
- Decision/Selection Process of Application Systems, Interchangeable Filling Systems
- Performance and Integrity Method Development and Testing of Systems (excluding Pre-Filled Syringes and Wearables)
- Formulation, Stability, and Delivery Challenges with Monoclonal Antibodies, Vaccines, Biosimilars, Antibody-Drug Conjugates, Oligonucleotides, Novel Therapies, including High Concentration, High Viscosity, Large Volume, Shear Sensitive, Oxygen-Sensitive, Temperature Sensitive
- Bubble Formation, Headspace, Gas Formation
- Testing of Pre-Filled Syringes
- Platform Approaches, Accelerating Market Access
- Usability Studies
- Injection Physiology
- Life Cycle Management
- Device Bridging Strategy and Risk Mitigation
- 3. Manufacturing and Quality Control
- New Equipment and New Technologies including Latest Developments in Fill-Finish and Assembly Equipment e.g., No-Touch Transfer Considering EU GMP Annex 1
- Total Product Quality, Zero Defect Challenges/Control Strategies
- Radionuclide Production and Delivery
- Visual Inspection (Manual/Automated)
- Primary and Secondary Packaging e.g., Labelling, Smart Labelling
- Track and Trace, Serialization, and Unique Container/Single Unit Identification
- Clinical Trial and Manufacturing Flexibility, Small Batches, Upscaling and Technology Transfer
- Automation and Digitalization
- Lyophilization in Syringes and Cartridges
- Contract Services/Best Practices
- Component Preparation, Decontamination, and Sterilization Methods
- Siliconization of Components, Silicone-Oil-Free Systems
- Needle Shapes, Handling & Preparation & Control Strategies
- Application of Artificial Intelligence in Primary Packaging, Fill-Finish, Device Assembly
- 4. Innovation in Components, Application Systems and Delivery Devices
- New and Sustainable Materials
- Autoinjectors and Pens
- Syringe and Cartridges Components
- Combination Products
- Pump Systems and Wearables
- Needle-Free Injections/Micro-Needles/Intradermal Injections
- Advanced Therapy Medicinal Products
- PFAS-Free Approaches
- 5. Smart Devices, Smart Packaging, and Digital Health Applications
- Device, Healthcare Provider, and Patient Interface
- Awarness of Patient Needs
- Improving Patient Adherence
- Roadmap for Implementation of New Technologies
- Application of Novel Technologies in Clinical Research and Commercialization
- Data Analytics, New Service Models
- Big Data, Data Mining, the “Transparent Patient” and Personal Data Protection
- 6. Stakeholder’s Needs
- Patient's First-Hand Experience
- E.g., From Internal Validation to Supply Chain Delivery - A Customer Perspective/Experience
- Caregivers, Healthcare Providers, Hospitals
- E.g., From Ampoules & Vials to Ready-To-Inject Systems - Benefits and Challenges Among Stakeholders
- Ease of Access to Medicines, Specific Needs in Delivery Devices & Packaging
- Human Factors, Usability, Handling, Functionality
- Patient-Led Device Design
- Novel Interaction between Patients and Healthcare Providers
- Care Management and Shift to Home Care Solutions
- Safety, Accidental Needle Stick Prevention
- Reduction of Cost, Reimbursement/Payer Aspects
- Patient's First-Hand Experience
- 7. Business Development and Marketing
- Emerging Markets
- Marketing Trends for Pre-Filled Syringes and Delivery Systems
- Infrastructure and Storage Issues
- Accessibility/Cost Considerations
- Technology/Competitive Advantage
- Speed to Market
- Suppliers and Industry Cooperation/Joint Projects/Collaboration Models
- 8. Re-Thinking Packaging and Devices for Sustainability Adaption
- Product Carbon Footprint Calculations/Life Cycle Assessments
- Solutions to Reduce Product Carbon Footprints including Net-Zero Products
- Circular Economy, Circular Packaging, and Circular Devices
- How to Establish an Industry Movement (vs. Individual Company Objectives)?
- Eco-Balance and Lifecycle Assessment Beyond Carbon
- Eco-Friendly Design/Material/Packaging/Supply Chain
- Design for Reuse, Recycling & Disposal Practices for Packaging and Devices
- Water, Waste, and Energy Reduction Programs including for Hazardous Drugs/Hazardous Medicinal Products
Poster Information
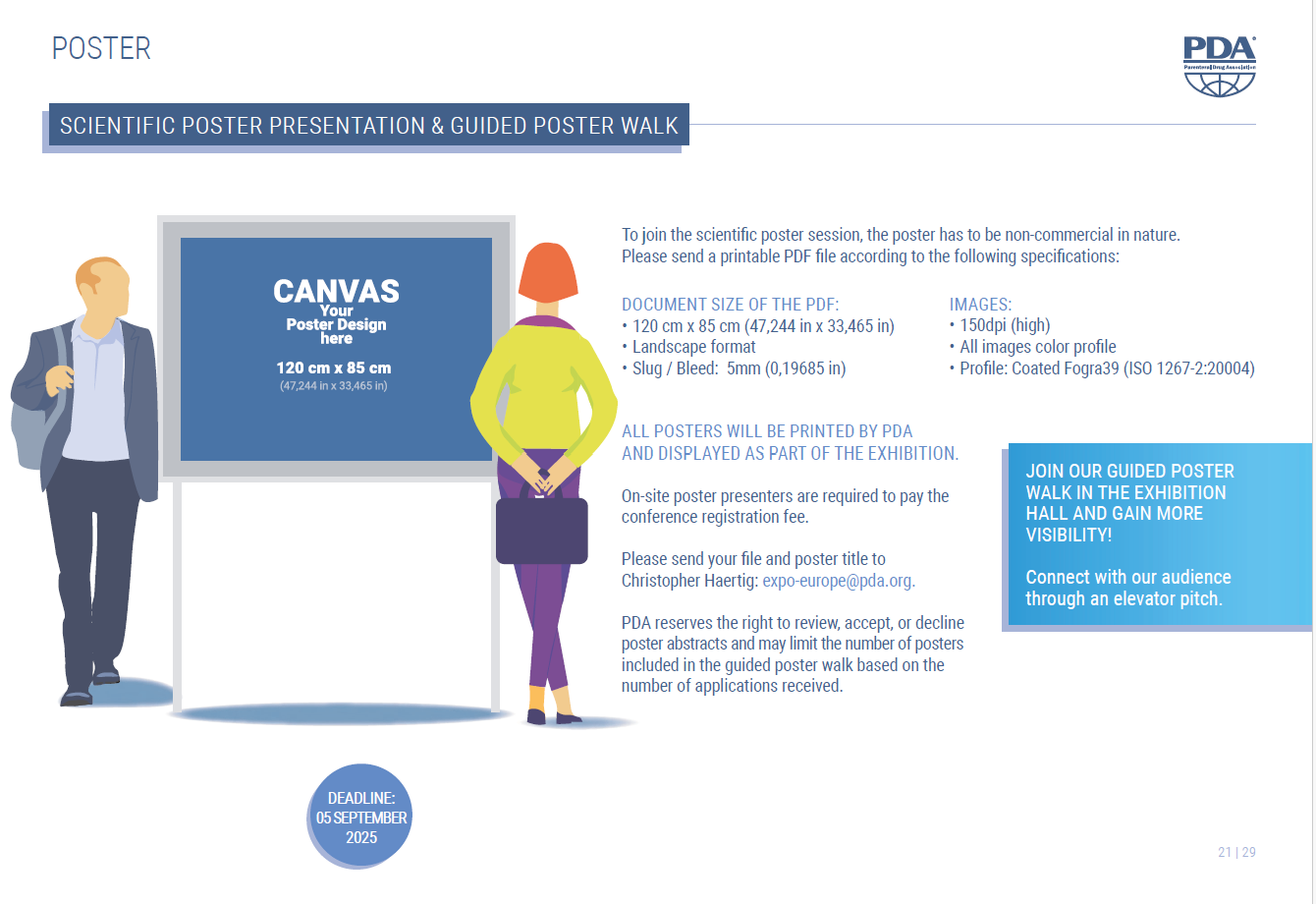
All posters will be printed by PDA and displayed as part of the exhibition.
Please send your final printable PDF file and poster title to expo-europe@pda.org.
Important
Poster presenters are required to pay a full conference registration fee.
Join our guided poster walk in our exhibition hall and gain more visibility. You will have the chance to engage with our audience!
Poster Display Dimensions
Please find the PDF dimension requirements below:
Exhibition and Sponsorship Opportunities
PDA is seeking vendors who provide products/services in support of this conference. Space on-site is limited and is on a first-come, first-serve basis.
Exhibition: 21-22 October 2025
To reserve your space, please contact Christopher Haertig at expo-europe@pda.org.