10 Practical Tips for Crisis Management in Pharma Manufacturing
Responding to the challenge of an invisible enemy requires lot of effort, strategy and structured execution. Even as pharma companies develop potential treatments and vaccines for COVID-19, they must also produce drug products for other conditions while remaining compliant under global GMPs.
Adhering to GMPs means being able to absorb unprecedented situations like the current crisis and manage them effectively without compromising product quality, safety, efficacy and purity. This could prove challenging due to instances of high absenteeism. Below are 10 ways pharmaceutical manufacturers can address manufacturing in a time of crisis:
1. Conduct an analysis of the company’s products/operations to discern needs for prioritized products and identify production that can be deferred.
2. Determine challenges that must be addressed (e.g., people, logistics, government regulations, supply chain, vendor support, internal quality systems, approvals, decision making, product quality risk and compliance standards).
3. Devise an emergency response plan (ERP) that includes a list for each function that directly and/or indirectly contributes to the stated objective. Also, determine when to activate and deactivate the plan.
4. Ensure that individual departments and relevant stakeholders are part of performing a step-by-step assessment on what operations can be deferred, what kind of data is available and how the data can support deferment decisions.
5. Develop a plan for deferments that lists each item to be deferred, supporting information, proposed actions, and documentation criteria. A simple 2/2 matrix table can be a useful tool (see Table 1 for an example).
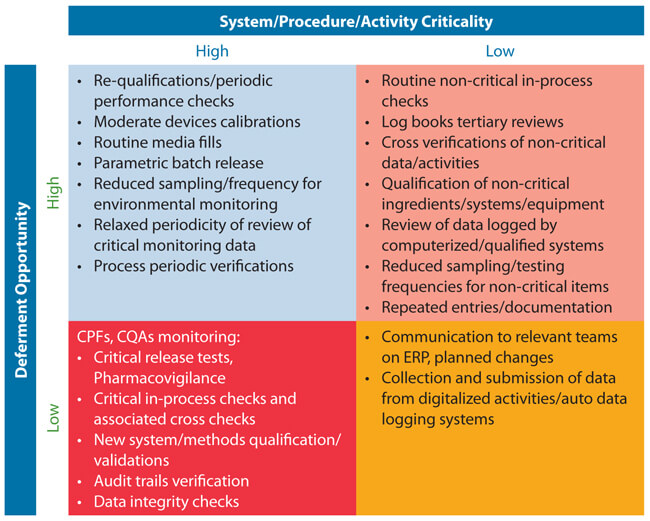 Table 1 Sample 2/2 Matrix Table
Table 1 Sample 2/2 Matrix Table
6. Plan how to bring deferred items back to normal system/procedure.
7. Communicate the plan extensively to all relevant personnel (the goal should be to ensure no one makes assumptions).
8. Train teams on emergency plan and planned changes that are going to be implemented as a part of ERP. Ensure that any critical departure from regulatory commitments are communicated to regulatory agencies. Note that the U.S. FDA requires ERP submissions for Medically Necessary Products include instructions for when will it be activated/deactivated, how it works, which risk assessment signs will be followed and how pending items (planned changes) are addressed in order to bring it back to the normal status.
9. Implement the ERP and increase monitoring on critical areas/items so that the following basic expectations are not compromised:
- Quality, purity safety and efficacy of the drug product
- Exceptions and associated procedures/methodologies are approved by Quality Unit.
- Each exception/planned change is supported by scientifically sound quality risk evaluation and is data oriented
- Documentation, checks and approvals are clearly defined and monitored
- Proper training and pertinent documentation
- Internal communication and communication to relevant regulators
10. Approve a plan for resuming to original procedures/practices at an appropriate time. Any additional testing/monitoring for the deferred activities to bring them back to normal must be supervised and cross checked and have clearly approved conclusions by Quality Unit. Be prepared with all relevant data for managing subsequent regulatory inspections. Post-pandemic, this could be an area of concern by inspectors.
Conclusion
Finally, it is apparent that the COVID-19 situation has brought to light the need for contingency planning and crisis management for pharmaceutical manufacturers. The ten steps outlined above can serve as the basis for a plan not just for the current situation but for future events.



 M. Satya Moturi, PhD, has over 25 years’ experience in the industry and his specialties include quality, regulatory affairs and aseptic operations. Currently, he is Senior Vice President, Quality, Regulatory and Scientific Affairs at Biological E. Ltd.
M. Satya Moturi, PhD, has over 25 years’ experience in the industry and his specialties include quality, regulatory affairs and aseptic operations. Currently, he is Senior Vice President, Quality, Regulatory and Scientific Affairs at Biological E. Ltd.