A Comprehensive Review of Regulatory Intelligence and Its Framework

The biopharmaceutical industry is a dynamic and rapidly evolving landscape that requires a thorough understanding of complex regulatory changes. Keeping track of the latest developments is critical for quality and regulatory professionals to remain compliant and mitigate risks.
With insights from regulatory agencies, industry associations, and other key stakeholders, regulatory intelligence is fundamental for staying up to date with the latest developments, patient safety, requirements, compliance, and product quality, according to the ICH Q10 Quality System guideline. The link between regulatory intelligence and a company’s change control process is key in providing the agility necessary to respond to new regulations. This article is meant to provide a comprehensive overview of regulatory intelligence and offer actionable insights and strategies for biopharmaceutical stakeholders, particularly manufacturers, to address these challenges.
Defining Regulatory Intelligence, Regulatory Surveillance, Regulatory Information, and External Engagement
According to the Drug Information Association’s Regulatory Intelligence Network Group, their definition of regulatory intelligence is “The act of gathering and analyzing publicly available regulatory information. This includes communicating the implications of that information and monitoring the current regulatory environment for opportunities to shape future regulations, guidance, policy, and legislation."
The last part of their definition mentions the “opportunities to shape future regulations, guidance, policy, and legislation.” At the Regulatory Intelligence Discussion Group, we believe the opportunities mentioned should be considered a separate aspect of regulatory intelligence identified as external engagement. We believe that a definition of regulatory intelligence should include regulatory surveillance and external engagement, both utilizing regulatory information to achieve their goals (See Figure 1). In this refined definition, regulatory intelligence is a multifaceted approach. It involves staying informed about regulatory changes and engaging proactively with regulatory authorities and stakeholders to influence and contribute to the evolving regulatory landscape.
With that being said, there are a few key areas to define:
- Regulatory intelligence
- Regulatory surveillance
- Regulatory information
- External engagement
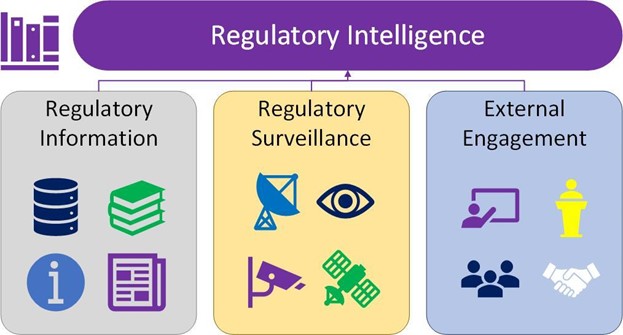 Figure 1 Regulatory Intelligence Breakdown
Figure 1 Regulatory Intelligence Breakdown
Regulatory intelligence: Regulatory intelligence is comprised of regulatory surveillance, regulatory information, and engagement. This includes the surveillance of new/updated regulatory information, evaluation of follow-up actions from the information, and engagement with stakeholders, such as regulatory authorities and industry associations. All of these are critical factors in ensuring a successful regulatory intelligence program.
Regulatory surveillance: The act of monitoring and gathering publicly available regulatory information. The regulatory information is then used to inform internal stakeholders of the potential strategies, action items, and change controls for their business operations.
Regulatory information: The publicly published/issued data are relevant to the biopharmaceutical industry. For example, this can include laws, acts, regulations, guidance, compendia, monographs, position papers, white papers, documents, news articles, presentations, alerts, databases, etc., from issuers such as regulatory agencies and industry associations.
External engagement: It is the discussion between stakeholders and the issuer of the document. Stakeholders can be individual companies, industry associations, or regulatory agencies that discuss a document with the issuer. External engagement can also be known as regulatory advocacy, policy, external liaison, and external affairs.
Frameworks for Regulatory Intelligence
A successful regulatory intelligence program starts with a firm foundation of surveillance of regulatory information. That information is processed for potential impact on the company’s business to ensure compliance. Once the company has firmly established a process for monitoring and processing regulatory information, a proactive approach to influencing the regulatory environment is the key element in external engagement.
Regulatory Information
There is a plethora of regulatory information in the world of regulatory intelligence. Regulatory authorities, industry associations, and individual contributors share information. For example, from 2018 to 2022, the U.S. FDA issued 771 guidances, where approximately 70% were final guidance documents, and an estimated 600 guidelines were issued by the European Medicines Agency EMA for the same time frame. This is an example of the amount of information from just two agencies. Because of the amount of information, we need to structure it.
We believe that regulatory information can be structured and categorized by content type (e.g., regulatory guidances, industry position papers, questions and answers, codified administrative law, blogs, or technical reports). It is important to note that different content types hold diverse levels of impact. For example, a blog provides interesting and sometimes useful information but typically does not evoke actionable items. On the other end of the spectrum, acts and laws mention requirements that are legally binding for the stakeholders to comply with. Content type can also be in distinct stages of publication (i.e., draft, final, and effective). Figure 2 illustrates grouped content types into generalized document categories and ranks them in order of impact.
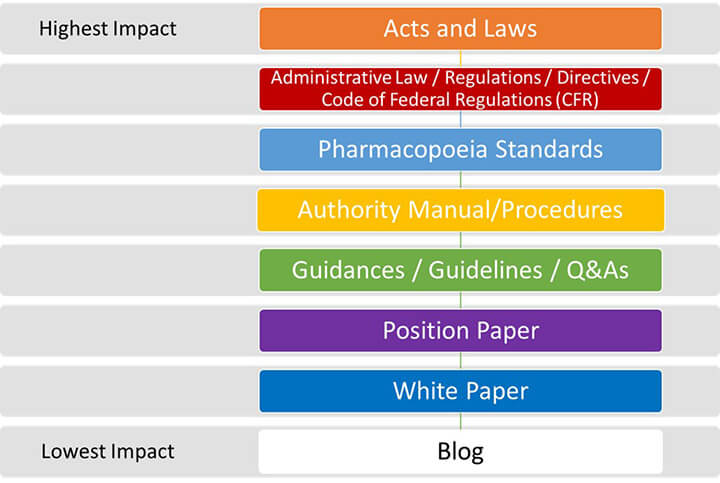 Figure 2 Document Content Ranking
Figure 2 Document Content Ranking
Regulatory Surveillance
Being able to evaluate the information and decide if action is needed is crucial for a business to remain compliant and competitive. There are four major activities for regulatory surveillance, and each activity can have a different maturity. This process has been simplified down to its core critical elements.
- The step-by-step process for regulatory surveillance starts with the need to monitor changes in relevant regulatory information.
- Capturing the relevant regulatory information in an organized manner.
- Distribution of information to stakeholders, including relevant subject matter experts (SMEs), for their risk-based evaluation and its impact on business.
- a. The SME is the point person to perform an impact assessment. If there is no impact, then the information can be stored and archived. However, if there is impact to the business, then appropriate measures need to be initiated. For example, a
deviation, change control, or implementation plan should be initiated with the appropriate stakeholders to ensure compliance.
- Tracking the progress of the stakeholders’ decisions from their evaluation to completion. This cycle is typically repeated for any type of information that is available, illustrated in Figure 3.
 Figure 3 Regulatory Information Process Flow
Figure 3 Regulatory Information Process Flow
External Engagement
Another aspect of regulatory intelligence is external engagement. Typically, this involves discussion between stakeholders and the issuer of the regulatory information.
New regulatory information is mainly drafted by regulatory agencies. These drafted documents provide an opportunity for stakeholder’s engagement. When the document is in the draft stage, the issuer typically provides a period of time for stakeholders to review the document and provide their feedback prior to the document’s final stage.
There are various levels of engagements with increasing maturity levels:
- One can comment on the issue as an individual. This is typically viewed as the least effective engagement.
- One can comment as a company directly to the issuer. This is typically a common approach for many pharmaceutical manufacturers.
- One can comment through an industry association. This is viewed as the most effective engagement approach because an industry association typically comprises many stakeholders from different companies, providing a unified voice to the issuer.
In our constantly evolving industry, new topics emerge that are not well defined. Therefore, a clearly defined guidance document may require a collaborative input from all stakeholders. A means of collaboration is a white paper or reflection paper issued. This is the greatest opportunity for all stakeholders to collaborate and shape the environment.
Additionally, external engagement can take a proactive approach. Stakeholders can directly reach out to regulatory authorities to request clarification of a topic within an existing regulatory framework. An example of this would be global regulators and industry associations working on ICH guidelines. Out of the different engagement types, this is viewed as the most mature. Other mature activities include various committees in external organizations and ad-hoc regulatory intelligence interest groups. Alternatively, regulatory authorities can issue white papers and reflection papers to gather stakeholder’s opinions to better shape future guidance/guideline documents. There are many different proactive engagement tactics at various levels.
Conclusion
Understanding regulatory intelligence and its strategies are imperative for a company, especially with the dynamic nature of our regulated industry. The second part of this article will focus on the different tools and program maturities of regulatory intelligence.





