A New Era for the PDA Journal
 With nearly eight decades since its establishment, the PDA Journal of Pharmaceutical Science and Technology (PDA JPST)
stand as one of the association's oldest and most valued membership benefits.
With nearly eight decades since its establishment, the PDA Journal of Pharmaceutical Science and Technology (PDA JPST)
stand as one of the association's oldest and most valued membership benefits.
The Bulletin of the Parenteral Drug Association was initiated as PDA’s scientific journal in 1947. After several name changes over the years, it was renamed the PDA Journal of Pharmaceutical Science and Technology in 1994, reflecting an enhanced emphasis on the progression of pharmaceutical science and technology, which is at the core of PDA’s mission.
Beyond its name change, the PDA JPST has made several notable advancements over the past two decades. These include the introduction of online submission and publication systems, the immediate release of articles upon editorial approval, and other initiatives.
Although these enhancements have improved both accessibility and the overall presentation of the PDA JPST, the Editorial Team has collaborated with the PDA Journal Editorial Board (JEB) to identify and implement additional opportunities to further strengthen the PDA JPST.
Expansion of the Editorial Team
The current Editorial Team includes Shanker Gupta (Editor in Chief), Ghada Haddad and Kurt Brorson (Associate Editors), Mike Sadowski (Managing Editor), and Whitney Alexis (PDA Publications Coordinator). Approximately three years ago, there were no additional active Editors to support Shanker which led to a backlog of unprocessed manuscripts. Since that time, Mike, Ghada and Kurt, each from different industry segments, have joined the Team with exceptional support from Whitney to reduce and eliminate the backlog. Prior to her retirement, PDA’s Marilyn Foster also played a key role in orientation of new Editors and in aiding them to address the backlog. The Editorial Team now has a diverse background and experience base, which provides full coverage of the technical disciplines that encompass the content in the PDA JPST.
Revitalization of the PDA Journal Editorial Board
The PDA Journal Editorial Board serves as stewards of the PDA JPST by offering scientific expertise, serving as authors and/or reviewers, providing recommendations on content, and actively promoting the PDA JPST through various networking channels to attract reviewers, authors, and high-quality manuscripts. The JEB consists of volunteers and has recently expanded from 20 to 48 members. New JEB members were thoughtfully selected from among highly engaged PDA members with strong technical backgrounds to effectively support PDA’s strategic goals in advancing manufacturing, quality, and regulatory science. In addition to broad technical expertise, the JEB features representatives from pharmaceutical and biopharmaceutical organizations from across the globe, ensuring balanced participation. For a complete list of the current 2025 JEB Roster, including each member’s name and affiliation, please refer to the JPST Editorial Board | PDA Journal of Pharmaceutical Science and Technology.
The goal is for JEB to meet quarterly, with two fully virtual meetings and two hybrid in-person/virtual meetings at PDA’s HQ or at a signature event. Currently, several active JEB task forces are underway. One task force is focused on increasing the visibility of the PDA JPST and its value proposition internally and externally to PDA. This task force sponsored the recent launch of the PDA JPST LinkedIn Group, which will serve as a communication channel for updates on the latest developments with the PDA JPST, to attract authors, and to highlight other member participation opportunities. You can stay informed by joining the PDA JPST LinkedIn Group.
Another task force is dedicated to enhancing the efficiency of the peer review process and has recently released a comprehensive guide outlining roles and responsibilities to assist reviewers, with the aim of reducing publication timelines while upholding the high quality of articles associated with PDA JPST. Additionally, a new task force will be established in November to select the 2025 Frederic D. Simon Paper of the Year, which will be awarded during PDA Week in March 2026. Further task forces can be created as future opportunities to improve and strengthen the PDA JPST are identified.
Launch of PeerTrack Manuscript Processing System
The manuscript processing system is central to the activities of the Editorial Team and authors, including paper submission, development of the reviewer competency roster, solicitation of reviewers, and administration of the review, revision, and acceptance processes for papers. Several issues and limitations with the BenchPress manuscript processing system became apparent and began to affect manuscript processing. In August 2024, the PeerTrack manuscript processing system was introduced as the replacement for BenchPress. Prior to PeerTrack's launch, the Editorial Team collaborated with the PeerTrack Development Team to discuss challenges with the old system that needed to be addressed and to ensure the new system's desired functional capabilities were fully implemented.
The PeerTrack system is very intuitive and incorporates many beneficial enhancements when compared to its predecessor, such as an Editor To-Do list dashboard with color-coded indicators for manuscript status and priority, advanced features for Personal Classifications to assist in peer-reviewed selection, enhanced options for searches and reports, a customizable due date alert system for authors and reviewers, and additional Editor functions aimed at simplifying manuscript processing by reducing unnecessary steps.
Since its implementation, PeerTrack has received positive user feedback and driven improvements in manuscript processing metrics, including reduced time from submission to acceptance.
Adoption of Metrics to Support the PDA JPST Mission
Since its implementation, PeerTrack has received positive user feedback and driven improvements in manuscript processing metrics. The PDA JPST is dedicated to disseminating scientific knowledge and technological advancements that advance quality and regulatory best practices for parenteral products. In response to ongoing industry developments, the Editorial Team and the JEB remain committed to attracting scientific articles that further these best practices, streamlining the manuscript review process, and upholding the high standards of excellence that have defined the PDA JPST for more than 80 years from submission to acceptance.
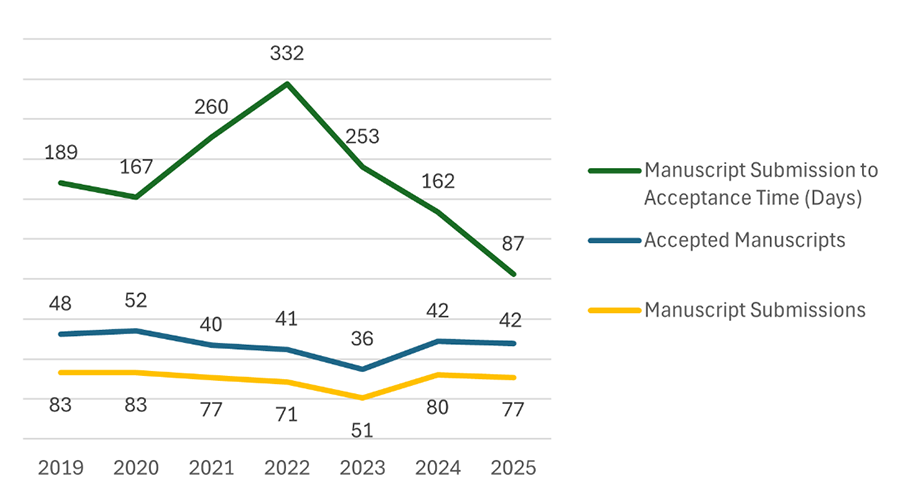
The following three metrics in Figure 1 have been adopted to assess and continuously improve performance in support of the PDA JPST’s mission: number of manuscripts submitted, number of manuscripts accepted, and the time period between manuscript submission and acceptance from 2019 to 2025.
Figure 1 demonstrates that manuscript submissions remained relatively consistent from 2019 to 2021. However, there was a notable decrease in submissions during 2022 and 2023, followed by a partial recovery in 2024 and 2025, although the numbers did not return to previous levels. The number of accepted manuscripts followed a similar trend to submissions, albeit at lower levels, which aligns with expectations, since not all manuscripts are accepted for publication.
The submission-to-acceptance time reached its longest time at 322 days in 2022, primarily due to challenges associated with BenchPress and other inefficiencies previously described. The decrease in manuscript submissions appeared to follow the increase in submission-to-acceptance time. This may partially explain the observed reduction in submitted manuscripts, although other potential factors, such as COVID, occurred during the same general timeframe. Since 2022, this time has steadily decreased, reaching 87 days in 2025. This shift coincides with the expansion of the Editorial Team, increased support from JEB and PDA Staff, and the introduction of the PeerTrack Manuscript Processing System, among other initiatives implemented by the Editorial Team. These factors have clearly contributed to significantly reduced submission-to-acceptance times.
PDA Community Support of the PDA JPST
The PDA JPST relies on the strength of its diverse membership, whose exceptional expertise, passion, and dedication drive scientific progress and the implementation of best practices in parenteral manufacturing, quality assurance, and regulatory science. In addition to the distinguished contributions of its authors, the PDA JPST’s success is a product of the commitment of volunteer members who conduct rigorous scientific peer reviews of submitted manuscripts.
Call for Authors
The PDA JPST is an internationally recognized source of peer-reviewed scientific and technical papers covering many topics of interest to our community, including:
- Pharmaceutical and biopharmaceutical manufacturing
- Sterile product production
- Aseptic processing
- Pharmaceutical microbiology
- Quality
- Packaging science
PDA JPST papers contribute to the advancement of scientific practices in alignment with PDA’s mission of connecting people, science, and regulation for the benefit of patients. Based on the summaries and data presented above, it has been observed that the PDA JPST’s submission-to-acceptance performance may have impacted manuscript submission rates. However, the information also indicates that issues affecting performance have been addressed, and ongoing evaluation using current and future metrics aims to further strengthen the PDA JPST. In addition to the improvements noted for the PDA JPST, the approaches and practices in our industry are rapidly advancing, so there has never been a better time to contribute as an author!
Authors can submit manuscripts to the PDA JPST—select “Instructions for Authors
Call for Reviewers
Volunteer reviewers play a critical role in the comprehensive, robust, and scientifically sound peer reviews that are essential to ensure the publication of high-quality manuscripts in the PDA JPST. Reviewers are recruited based on their areas of expertise, now known as Personal Classifications, which are entered into PeerTrack, a system designed and developed to support an efficient review process. In addition to supporting the success of the PDA JSPT as reviewers, individuals also benefit from early access to state-of-the-art advancements in their field when reviewing manuscripts prior to publication. Reviewers have opportunities to broaden their professional networks through collaborative interactions with authors while enhancing the quality of submitted manuscripts. The valuable contributions of reviewers are acknowledged with a token of appreciation and official recognition in the first issue of the PDA JPST each year.
Everyone can be a reviewer, and it takes less than 5 minutes to register—select “Instructions for Reviewers” at the PDA JPST.
Note: Authors, Reviewers and interested parties can contact the journal staff for assistance at journal@pda.org.
Summary
The PDA Journal of Pharmaceutical Science and Technology remains one of our most longstanding and significant membership benefits. Over the past three years, PDA has prioritized strengthening the PDA JPST through expansion of the Editorial Team, revitalization of the JEB, investment in the PeerTrack Manuscript Review System, and the establishment of performance metrics to drive ongoing improvement. We extend our sincere appreciation to the authors, reviewers, JEB members, PDA staff, and, above all, the readers, whose collective contributions have positioned the PDA JPST as the leading international publication in advancing scientific discovery and best practices for parenteral products. We strongly encourage all members to consider contributing to the continued success of the PDA JPST as authors or reviewers.



 Michael Sadowski, Sterilexcellence, has 30 years of experience with drug and device sterilization with a variety of sterilization modalities. In addition to participation on the Task Force for the revision of PDA TR No. 1 on Moist Heat Sterilization, he was the Chair of the Task Force for the revision of the PDA TR No. 30 on Parametric Release. Mike previously served as Treasurer, Secretary and Director on the PDA Board of Directors and member of the PDA Scientific Advisory Board and currently serves an Associate Editor for the PDA Journal and Co-Chair of AAMI WG 03 on Moist Heat Sterilization. He has successfully aligned industry and regulatory agencies to shape best practice and continues to actively publish and contribute presentations and training sessions on moist heat sterilization and parametric release. He is frequently sought as an expert speaker by global industry and regulatory Sterility Assurance professionals. Mike received his BS Degree in Microbiology from Purdue University.
Michael Sadowski, Sterilexcellence, has 30 years of experience with drug and device sterilization with a variety of sterilization modalities. In addition to participation on the Task Force for the revision of PDA TR No. 1 on Moist Heat Sterilization, he was the Chair of the Task Force for the revision of the PDA TR No. 30 on Parametric Release. Mike previously served as Treasurer, Secretary and Director on the PDA Board of Directors and member of the PDA Scientific Advisory Board and currently serves an Associate Editor for the PDA Journal and Co-Chair of AAMI WG 03 on Moist Heat Sterilization. He has successfully aligned industry and regulatory agencies to shape best practice and continues to actively publish and contribute presentations and training sessions on moist heat sterilization and parametric release. He is frequently sought as an expert speaker by global industry and regulatory Sterility Assurance professionals. Mike received his BS Degree in Microbiology from Purdue University.  Dr.
Shanker Gupta has been a member of the Journal Editorial Board since 2019 and, most recently, has served as an Associate Editor, helping evaluating and managing JPST submissions. He has been a PDA member for more than 30 years. Dr. Gupta has worked with the National Institutes of Health for almost 20 years, currently as a senior scientist in the Division of Cancer Prevention of the National Cancer Institute and consulting with the Vaccine Research Center of the National Institute of Allergy and Infectious Diseases. At NIH, he has been responsible for managing multiple translational projects designed to generate clinical product for use in Phase I and Phase 2 clinical trials. Dr. Gupta also collaborates with contract teams and actively participates in the design and development of formulations of several promising agents that have resulted in patent filings. He has supported over 40 IND submissions. Dr. Gupta holds a BS (Hons) in pharmacy from the University of Florida and a PhD in pharmaceutical chemistry from the University of Michigan, Ann Arbor. Prior to joining NIH, he was a project leader in sterile products development at Abbott Laboratories and filed the IND for the expanded use of a lung surfactant product (Survanta®), which was the first product of its kind for neonatal respiratory distress syndrome. Gupta has published more than 20 papers in various peer-reviewed journals and contributed to books.
Dr.
Shanker Gupta has been a member of the Journal Editorial Board since 2019 and, most recently, has served as an Associate Editor, helping evaluating and managing JPST submissions. He has been a PDA member for more than 30 years. Dr. Gupta has worked with the National Institutes of Health for almost 20 years, currently as a senior scientist in the Division of Cancer Prevention of the National Cancer Institute and consulting with the Vaccine Research Center of the National Institute of Allergy and Infectious Diseases. At NIH, he has been responsible for managing multiple translational projects designed to generate clinical product for use in Phase I and Phase 2 clinical trials. Dr. Gupta also collaborates with contract teams and actively participates in the design and development of formulations of several promising agents that have resulted in patent filings. He has supported over 40 IND submissions. Dr. Gupta holds a BS (Hons) in pharmacy from the University of Florida and a PhD in pharmaceutical chemistry from the University of Michigan, Ann Arbor. Prior to joining NIH, he was a project leader in sterile products development at Abbott Laboratories and filed the IND for the expanded use of a lung surfactant product (Survanta®), which was the first product of its kind for neonatal respiratory distress syndrome. Gupta has published more than 20 papers in various peer-reviewed journals and contributed to books. Kurt Brorson PhD, Parexel International, has over 26 years of FDA experience in CBER and CDER, specializing in CMC review, facility assessment, and compliance for BLAs, DMFs, and INDs. As a former Lab Chief in CDER’s Office of Biotechnology Products, he led a respected program on viral safety of biotechnology products and contributed to FDA policy, guidance, and training development. A recognized expert in viral clearance and biopharmaceutical safety, Kurt holds a PhD in Molecular Biology from Caltech and a BA in Biology from the University of Chicago.
Kurt Brorson PhD, Parexel International, has over 26 years of FDA experience in CBER and CDER, specializing in CMC review, facility assessment, and compliance for BLAs, DMFs, and INDs. As a former Lab Chief in CDER’s Office of Biotechnology Products, he led a respected program on viral safety of biotechnology products and contributed to FDA policy, guidance, and training development. A recognized expert in viral clearance and biopharmaceutical safety, Kurt holds a PhD in Molecular Biology from Caltech and a BA in Biology from the University of Chicago. Ghada Haddad, PhD, Kite Pharma, is the Head of Quality Systems and Quality Processes at Kite Pharma (a Gilead Company), bringing over 24 years of experience in biotechnology and pharmaceuticals. She leads global teams across Quality Systems, Sterility Assurance, Risk Management, Data Science, Auditing, and Compliance. Ghada holds a PhD in Pharmaceutical and Regulatory Science, an MBA, and a BS in Chemistry. Formerly with Merck and Genentech, she is recognized for driving quality transformation and digital innovation and actively contributes to industry groups including PDA, ICH, ISPE, and PQRI.
Ghada Haddad, PhD, Kite Pharma, is the Head of Quality Systems and Quality Processes at Kite Pharma (a Gilead Company), bringing over 24 years of experience in biotechnology and pharmaceuticals. She leads global teams across Quality Systems, Sterility Assurance, Risk Management, Data Science, Auditing, and Compliance. Ghada holds a PhD in Pharmaceutical and Regulatory Science, an MBA, and a BS in Chemistry. Formerly with Merck and Genentech, she is recognized for driving quality transformation and digital innovation and actively contributes to industry groups including PDA, ICH, ISPE, and PQRI.