A Successful Q-Model for New mRNA Line Commissioning & Qualification: Pt. 1

Contract development and manufacturing organizations (CDMO) have become a crucial partner in fighting the ongoing COVID-19 pandemic. With the successful commercialization of many mRNA vaccine technologies and the newly developed traditional vaccines during the pandemic, there is a need for incremental commercial-scale isolator technology-based vaccine filling lines (1).
Responsible experienced manufacturers have initiated expansion projects on an accelerated path while continuing to deliver lifesaving vaccines from their existing capacities. The parallel efforts to bring state-of-the-art fill lines are expected to secure the vaccine supply chain for ongoing and future pandemics, a critical mandate. The key objectives include adding classified areas for activities like gowning, component preparation, sterilization, washing, tunnel depyrogenation, isolators, high-speed filling, capping, automatic inspection, cold chain storage, decontamination, packaging, labeling, quality, microbiology and sterility testing, utility supply, and support services.
The equipment and facility design efforts focus on implementing current technologies to deliver a high level of automation and data monitoring, while meeting Biosafety Level Class 2 levels. Integrating the patient safety, quality, and productivity focused newer technologies into existing quality systems needs special attention.
This two-part article highlights some of the successful quality and compliance strategies applied during commissioning and qualification of a high-speed mRNA fill-finish line. Part 1 discusses the steps for turning organizational ideas (tacit to explicit) into practical commissioning and qualification requirements while meeting regulatory and industry guidelines.
Setting the Standard
Commissioning and qualification projects involving adoption of cutting-edge technologies at an existing site requires an engineering planning phase to plan for the demolition, reconfiguration, installations, buildout, and decommissioning. Equal weightage is provided to review the impact of the changes and incorporation into the existing quality management systems. Development of detailed fit-for-purpose user requirement specifications (URS), design specifications, functional specifications, hardware specifications, software specifications, and commissioning and qualification plan (CQV) requires full involvement of the quality unit, especially for sites with existing operation. ASTM E2500-20: Standard Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing Systems and Equipment concepts are yet to be realized in the industry (2). The guidance stresses the importance of application of risk management and incorporation of quality by design to ensure that the critical product/process attributes are built into the equipment/system. The heightened level of Stage 1 activities as expected in ASTM E2500 are not always possible, especially with CDMOs where product/process information is not readily available for upfront equipment trial runs. The most common approach is technology adoption to fit the planned capacities rather than its development. A hybrid approach is therefore ideal for such projects to avoid redundant testing. It supports sound science-and risk-based decision-making that drives the specification creation, design and verification. ASTM E2500 is referenced in U.S. FDA’s Guidance for Industry: Process Validation: General Principles and Practices, authenticating FDA’s agreement with its content (3).
With the current need for accelerated fill-finish manufacturing capacity expansions, such as that for mRNA products, some additional measures can help in delivering the desired outcomes. Contrary to the belief of having a reduced level of quality involvement, upfront full involvement of quality and product/process subject matter experts (SME) is required for such projects. There are four main reasons for this:
- Determining Business Fit: For CDMOs it is crucial to get input from customer facing groups such as business development teams regarding the potential project types, the technologies in demand, and adjustable solutions that help provide the needed flexibility when the product is not identified.
- Initial Quality Risk Management (QRM): Application and alignment on risk identification consistent with ASTM and development of a CQV plan based on the identified risks needs extensive quality and SME input. An incomplete risk determination exercise may result in exclusion of the required test cases. Skipping tests at the commissioning stage can contribute to qualification stage failures. Equipment or software test failures during qualification, or process validation will also cause project delays with added cost to quality.
- Leading to a URS: While additions and enhancements are expected, further changes to the fundamental requirements can cause unexpected delays in execution as well as pose compliance challenges. Therefore, it is not ideal to project URS as an immediate milestone for such projects. Instead, building an agile and well-balanced quality–SME–vendor team with defined responsibilities is the critical milestone. The quality unit can identify and document regulatory/internal quality expectations; SMEs can gather and document product/process knowledge. The knowledge repository to develop URS can include information from the multiple brainstorming sessions, internal and external, held to develop requirements (Figure 1).
Developing design, functional, hardware and software specifications are important tasks as they need to consider product/process requirements and address any regulatory trends, for example, manufacturing operations data integrity and data governance. Full involvement of the quality unit, SMEs and the equipment vendor are imperative during this stage.
Management expectations need to be set such that the completion of the initial QRM, the activities prior to a URS and the development of specifications are the first set of mandates. The upfront development of knowledge and understanding of the equipment requirements forms the basis for establishing a manufacturing control strategy that results in products with the desired quality attributes. Where supporting data is not available for decisions, planned experiments or trial runs at the equipment vendor’s site may be employed to generate supporting evidence. The early quality involvement strategy also supports elimination of any repetitive tests, minimizing qualification stage activities, and reduces the potential for failures since the documented evidence and confidence/maturity level has already been achieved.
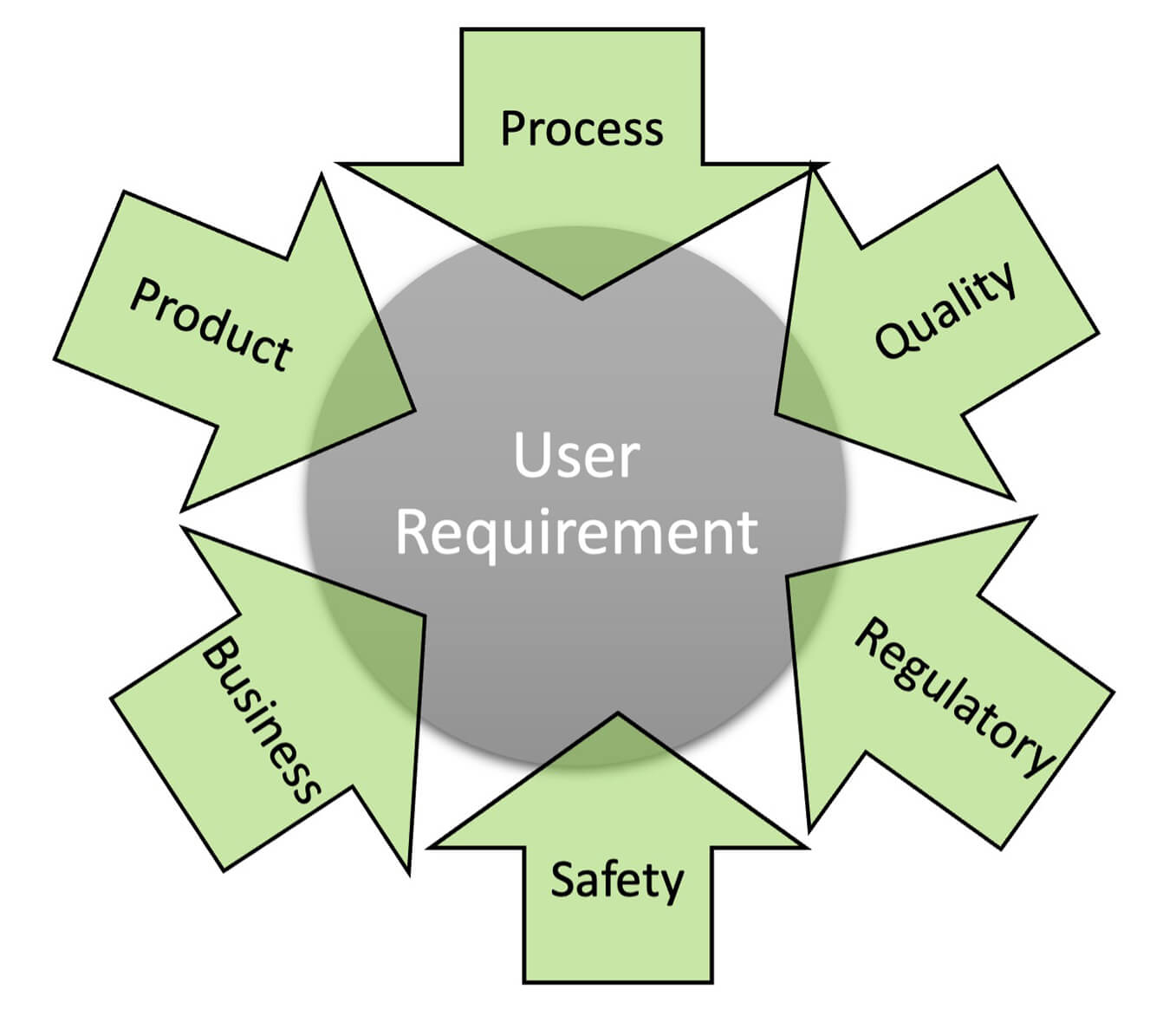 Figure 1 Knowledge Prerequisites
Figure 1 Knowledge Prerequisites
FDA guidances, EU GMP guidelines, ASTM standards and other regulatory guides are nonprescriptive on how to perform factory acceptance tests and site acceptance tests, develop specifications or designs or validate installation, operational and performance qualifications. This necessitates the need to align with industry best practices. Table 1 provides some of the applicable industry guidelines that need to be considered while planning for an accelerated mRNA manufacturing line. Adequate time needs to be spent developing a CQV plan for integrating the regulatory expectations and the practically applicable industry guidelines. Involving a quality and compliance expert is key at this point as the workflow, CQV stage-gate requirements, determination of the risk assessment tool and stages of updating the risk assessment (upon generation of new data) will be defined in the CQV plan.
FDA guidance, EU GMPs, ASTMs, and other regulatory guidelines are nonprescriptive on how to perform factory acceptance tests (FAT), site acceptance tests (SAT), develop specifications, design development, qualification (IQ, OQ, PQ) etc. This necessitates the need to align with the industry best practices. Table 1 provides a pointer towards some of the applicable industry guidelines that need to be reviewed while planning for an accelerated mRNA manufacturing line. Once assessed, the compliance to standards needs to be documented (see Table 2 for an example). Adequate time needs to be spent while developing a CQV plan for integrating the regulatory expectations and the practically applicable industry guidelines. Involving the quality and compliance expert is key since the workflow, CQV stage gate requirements, determination of the risk assessment tool, and the stages of updating the risk assessment (upon generation of new data) are to be defined in the CQV plan.
|
Industry Organization
|
Guideline
|
|---|---|
| PDA | Technical Report No. 54-5: Quality Risk Management for the Design, Qualification, and Operation of Manufacturing Systems |
| Technical Report No. 3: Validation of Dry Heat Processes Used for Depyrogenation and Sterilization | |
| Technical Report No. 61: Steam in Place | |
| Technical Report No. 22 (Revised 2011): Process Simulation for Aseptically Filled Products | |
| Technical Report No. 1, Revised 2007: Validation of Moist Heat Sterilization Processes: Cycle Design, Development, Qualification, and Ongoing Control | |
| Technical Report No. 48: Moist Heat Sterilizer Systems: Design, Commissioning, Operation, Qualification and Maintenance | |
| Technical Report No. 34: Design and Validation of Isolator Systems for the Manufacturing and Testing of Health Care Products | |
| Technical Report No. 4: Design Concepts for the Validation of a Water for Injection System | |
| Technical Report No. 70: Fundamentals of Cleaning and Disinfection Programs for Aseptic Manufacturing Facilities | |
| Technical Report No. 13 (Revised): Fundamentals of an Environmental Monitoring Program | |
| Technical Report No. 84: Integrating Data Integrity Requirements into Manufacturing & Packaging Operations | |
| Technical Report No. 60: Process Validation a Lifecycle Approach | |
| Points to Consider for Aseptic Processing, Parts 1 and 2 | |
| ISPE | Baseline Guide Vol 5: Commissioning & Qualification |
| Good Practice Guide: Practical Implementation of the Lifecycle Approach to Process Validation | |
| Good Practice Guide: Applied Risk Management for Commissioning and Qualification | |
| Good Practice Guide: Process Gases | |
| BioPhorum | Environmental Monitoring (EM): A harmonized risk-based approach to selecting monitoring points and defining monitoring plans |
|
Guideline: ISPE Baseline Guide Vol. 5: Commissioning and Qualification
|
||
|---|---|---|
| Sections | Meets/ Not Applicable/ Alternate Strategy | Reference Document/s |
| 2. User Requirements Specification | ||
|
2.2 Development of the User Requirements Specification |
Meets | VMP
SOP 0455 Site URS Template |
|
2.3 Approval of User Requirements Specification and Changes Post-Approval |
Meets | SOP 0455 |
|
2.4 Legacy Systems |
Risk assessment required to determine impact to legacy systems used for the new line | Risk Assessment #2136 Initiated |
| 3. System Classification | ||
| 4. System Risk Assessment | ||
| 5. Design Review and Design Qualification | ||
| 6. C&Q Planning | ||
| 7. C&Q Testing and Documentation | ||
| 8. Acceptance and Release
|
||
| 9. Periodic Review
|
||
| 10. Vendor Assessment for C&Q Documentation Purposes | ||
| 11. Engineering Quality Process | ||
| 12. Change Management | ||
| 13. Good Documentation Practice for C&Q | ||
In addition to risk management and leveraging criteria, Part 2 of this article will discuss the integration considerations for facilities with existing operations.
References
- Xie, W, Chen, B, and Wong, J. “Evolution of the market for mRNA technology,” Nature Reviews/Drug Discovery 2021, Oct, Vol. 20, p. 735-36. https://www.nature.com/articles/d41573-021-00147-y.
- American Society for Testing and Materials. ASTM E2500-20 Standard Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing Systems and Equipment. https://www.astm.org/e2500-20.html.
- Pazhayattil, A.B, Sayeed-Desta, N, Chen, S, and Ingram, M. “A Semi- Quantitative Risk Assessment Methodology Fit for Bio-Pharmaceutical Lifecycle Stages,” PDA J Pharm Sci Tech 2020, 75 (6): https://journal.pda.org/content/early/2020/02/14/pdajpst.2019.010173.



 Ahmed Elsaid, is a Quality Senior Director at Emergent Biosolution Rockville site. Ahmed has over 21 years experience in the pharmaceutical industry as a quality leader in parenteral manufacturing. He has diverse experience working in four different continents addressing the different regulations and managing projects in different phases of the life cycle. His experience spans multiple disciplines including quality systems, aseptic processing, validation, analytical and process development, commissioning, and qualifications.
Ahmed Elsaid, is a Quality Senior Director at Emergent Biosolution Rockville site. Ahmed has over 21 years experience in the pharmaceutical industry as a quality leader in parenteral manufacturing. He has diverse experience working in four different continents addressing the different regulations and managing projects in different phases of the life cycle. His experience spans multiple disciplines including quality systems, aseptic processing, validation, analytical and process development, commissioning, and qualifications. Ajay Babu Pazhayattil, has been providing strategic direction and leadership is his roles with brand, generic, biosimilar, and CDMO organizations. He is currently Vice President, Scientific and Regulatory Affairs at Capcium Inc., Montreal. Ajay is an industrial pharmacist successful in delivering tangible results across segments of pharmaceutical and biopharmaceutical operations. He is a contributing author, speaker, committee lead and influencer in the industry. Ajay has been part of developing and delivering regulatory acceptable product, process remediation strategies.
Ajay Babu Pazhayattil, has been providing strategic direction and leadership is his roles with brand, generic, biosimilar, and CDMO organizations. He is currently Vice President, Scientific and Regulatory Affairs at Capcium Inc., Montreal. Ajay is an industrial pharmacist successful in delivering tangible results across segments of pharmaceutical and biopharmaceutical operations. He is a contributing author, speaker, committee lead and influencer in the industry. Ajay has been part of developing and delivering regulatory acceptable product, process remediation strategies.