A Successful Q-Model for New mRNA Line Commissioning & Qualification: Pt. 2

The role of a contract development and manufacturing organization (CDMO) in bringing lifesaving vaccines to market has become critical with both current and future pandemic preparedness (1). The facility expansion projects intended to create adequate capacity to meet the needs of domestic markets have become a critical government-backed initiative worldwide (2). Today, commissioning and qualification (CQV) efforts require certain special considerations that factor in the emergence of advance automation technologies, the evolution of machine learning/artificial intelligence, and the involvement of cross-functional groups from around the world working remotely. The key objectives of commissioning and qualification of an mRNA line include adding classified areas for activities like gowning, component preparation, sterilization, washing, tunnel depyrogenation, isolators, high-speed filling, capping, automatic inspection, cold-chain storage, decontamination, packaging, labeling, quality, microbiology and sterility testing, utility supply, and support services. The equipment and facility design efforts focus on implementing technologies to deliver a high level of automation and data monitoring, while meeting Biosafety levels. Integrating patient safety, quality, and productivity focused newer technologies into existing quality systems needs special attention.
Part 1 of the series detailed the systematic steps followed to turn organizational ideas into practical commissioning and qualification requirements (tacit to explicit) while meeting global regulatory/industry requirements. Part 2 discusses the risk management strategies, development of leveraging criteria, and specific integration considerations for facilities with existing operations.
Facilities with existing operations need to assess the impact of adding a new line or equipment. In certain cases, utilities, operators, test instruments and procedures are common; the impact can be assessed prior to initiation of the CQV project, and adequate control measures can be put in place as part of the CQV plan. The utility capacities, adequacy of the number of test instruments and operator training are typically considered, as is the need for new procedures. The impact to existing operations during construction, such as utility supply, personnel and material flow, containment and cross-contamination during construction, microbial flora, and alarm management, especially need to be kept in mind (Figure 1).
Change control is a good tool for documenting the impact and tracking mitigation actions where temporary measures and revisions might be needed based on the progress of construction and project plans. The quality unit can also independently assess the impact of adding a new line to the quality management system. Addressing the gaps before construction begins will support a smooth execution. The introduction of a new process/line has an impact on existing facilities, utilities, and systems. Decisions on the new line, such as those about construction materials, design, construction management and installation, need to be made while considering existing qualified amenities. Including a design verification document that covers the existing facility, equipment and utilities is a good practice for verifying that each of the relevant systems has been reviewed and has demonstrated the capability to meet process requirements (3).
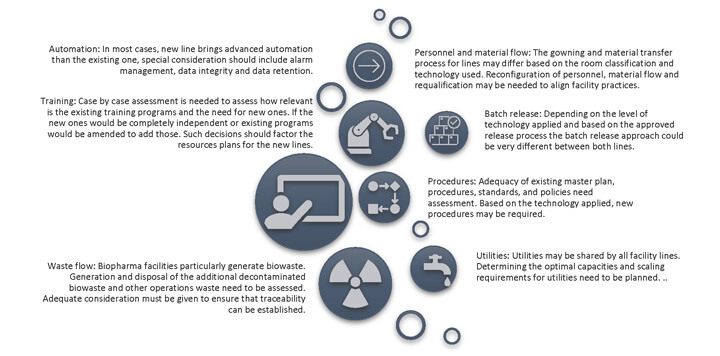 Figure 1 Integration Considerations at the Earlier Stages of a CQV Project (Integration with Existing Line)
Figure 1 Integration Considerations at the Earlier Stages of a CQV Project (Integration with Existing Line)
A CQV project is only successful when the manufacturing operations flawlessly meet process performance qualification (PPQ) requirements according to the U.S. FDA process validation guidance, EU GMP Annex 15, and other regulatory guidances. A matrix should be developed to count and cost the additional downstream work needed due to the lack of CQV data-gathering; this is typically performed by a manufacturing science and technology team. The additional activities may include identifying initial set-up run parameters, operating ranges and automation control parameters. Therefore, it is ideal to dismantle the CQV project team only after a successful PPQ has been completed, though that might take place later for CDMO organizations. Communicating the CQV project success will assure early involvement of the quality unit, subject matter experts and vendors.
Risk Management
Both ASTM and ISPE recommend applying a risk-based approach to drive activities. This necessitates performing an initial risk assessment at the onset based on literature, vendor knowledge and available product/process information. ICH Quality Guideline Q9: Quality Risk Management advocates using a science-based risk evaluation to protect patient safety and ensure the level of the risk management effort is commensurate with the level of risk. This requires choosing appropriate risk assessment tools to be employed during various stages. Such simple techniques as flowcharts and maps, fishbone diagrams (Ishikawa diagrams) or check sheets, fault tree analysis, failure mode effects (criticality) analysis, preliminary hazard analysis, hazard operability analysis, and hazard analysis and critical control point can be applied (4). These tools are appropriate when minimal data is available at the early stages. However, use of a systematic, data-driven risk-assessment tool is recommended following generation of sufficient equipment/product/process/design-of-experiment data (5). Figure 2 provides the CQV stages where a risk assessment should be performed based on the available incremental new data.
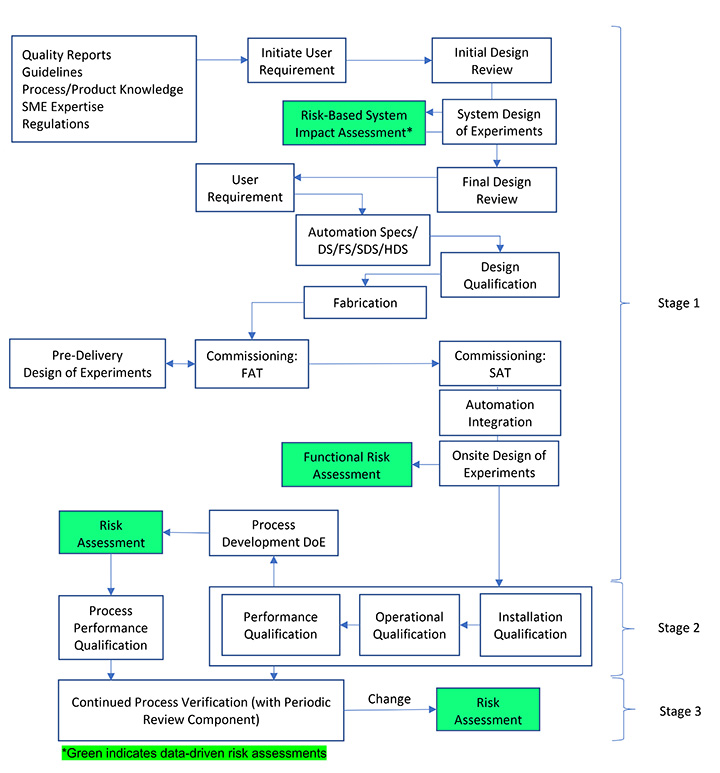 Figure 2 Risk Assessment Lifecycle – Commissioning and Qualification. Adapted from Pazhayattil (2021, Fig. 2) (6)
Figure 2 Risk Assessment Lifecycle – Commissioning and Qualification. Adapted from Pazhayattil (2021, Fig. 2) (6)
The draft revisions to ICH Q9 mandate decision-makers minimize subjectivity in risk assessment (7). Currently in public consultation, it states that subjectivity can be introduced using tools with poorly designed risk-scoring scales. Thus, justifying the risk-assessment tool being used at each stage of CQV is required to meet the new expectations.
Execution, Review, Approval and Leveraging
CQV activities and the associated data generated are perpetual in nature. This documentation will be routinely retrieved and presented during inspections that take place throughout the lifecycle of the facility. A review of U.S. FDA warning letters from 2012 to 2019 shows that 55% of the issues noted were related to data integrity. Additionally, 45% of those data-integrity observations were related to production operations data (8). Hence, special focus needs to be provided on continual training for good documentation practices (GDP), data governance and data integrity. This is even more important for fast-paced projects involving multiple CQV vendors.
The CQV plan needs to clearly identify the parties responsible for execution. If certain portions of the testing are to be performed in the presence of the equipment vendor or external SME, the requirements need to be defined. ASTM E2500-20: Standard Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing Systems and Equipment supports a risk-based qualification where, by leveraging the commissioning, early development activities are allowed to support qualification status. Previous credible experience with sufficiently similar processes can also be helpful. The test outcome and documentation adequacy must be assessed prior to leveraging, however. Therefore, though the downstream qualification protocol allows for leveraging, the preapproval of leveraging test cases must consider the test outcome and documentation compliance. The quality unit may want to develop a checklist (Table 1) that ensures those executing the CQV are cognizant of adhering to right-first-time principles.
The leveraging decision needs to be commensurate with various aspects of ASTM E2500: Has a formal risk assessment been completed and updated to be current? Have effective control measures been identified and implemented? Has a qualification protocol been developed that captures the identified risks? Involving the quality unit early on during the commissioning is strongly suggested, especially when designing the testing plan, amending any tests and managing any potential test failures. This involvement makes sure that the quality unit concurs with the commissioning approach in a way that could facilitate leveraging decisions and minimize any test-case repetitions.
| Description | Document/Circle One |
|---|---|
|
Document being leveraged: _________________________________ Protocol #: ______________ Stage: Commissioning / Qualification |
|
| Number of tests cases being leveraged | |
| Post-execution risk assessment completed? | Yes / No |
| Overall system risk rating | High / Medium / Low |
| Number of test failures in the study | |
| If failures observed, were corrective actions effective and retested? | Yes / No / NA |
| Open changes | Yes / No |
| Number of data integrity observations from document review | |
| Number of protocol errors observed | |
| Number of GDP execution errors | |
|
Criteria – Leveraging for Commissioning All test failures need to be adequately addressed and retests passed. There are no open changes, data integrity observations. There are no more than 2 protocol generation errors. There are no more than 5 GDP errors. |
|
|
Criteria – Leveraging for Qualification An updated risk assessment is complete post-execution. There are no more than 2 test failures for medium- and low-risk systems, and 0/ failures for high-risk systems. All test failures need to be adequately addressed and retests passed. There are no open changes, data integrity observations. There are no protocol generation errors. High-risk systems: There are 0/ execution GDP errors. Medium-/Low-risk systems: There are no more than 2 GDP errors per test case, and no more than 2 test cases with GDP errors can be leveraged. |
|
| Post-Execution Document Leveraging Recommendation | Yes / No |
Note: The above review supports preapproval of test-case leveraging.
Conclusion
An ideal CQV execution phase for a fast-paced project would need on-floor support from the quality unit so a cursory document review can take place in tandem. This strategy supports smooth execution and mitigation of potential issues in a contemporaneous manner. The review process can be streamlined in such a way that there is adequate time allocated for protocol generation and the drafting is completed only after equipment-vendor knowledge transfer is complete. Where possible, development of protocols can be scheduled post vendor site or on-site trial runs to understand the nuances of the new system or equipment. The right-first-time matrix is to be applied to the protocols. The leveraging checklist can act as a data-gathering mechanism for right first time.
The quality of the executed protocols or summary reports needs to be assessed based on the number of GDP, technical and data-integrity errors observed during the review process. The project management team must establish the CQV project success criteria and continually measure results. A fast-paced CQV project with a defined performance matrix supports management in making swift, informed decisions when needed. Facilitating ad hoc brainstorming sessions can identify further opportunities to accelerate the timeline. Constant communication that provides status clarity is a critical aspect, especially when some of the activities are performed remotely due to the pandemic restrictions. Considering the pace of the project, resource shortages need to be anticipated. The timelines of such projects are strongly driven by supply demands and patient needs. Having an agreed set of key performance indicators is crucial to help define the capacity of different teams, to measure progress, to address execution concerns, and to train and facilitate the prioritization at each site.
References
- Guzman, Joseph. “Bill Gates, who predicted the pandemic, names the next two monster disasters that could shake our world.” Interview on The Hill: Changing America, February 11, 2021. https://thehill.com/changing-america/well-being/538426-bill-gates-who-predicted-the-pandemic-names-the-next-two-monster/.
- Lander, Eric S and Sullivan, Jacob J, Eds. “American Pandemic Preparedness: Transforming Our Capabilities.” The White House, September 3, 2021. https://www.whitehouse.gov/wp-content/uploads/2021/09/American-Pandemic-Preparedness-Transforming-Our-Capabilities-Final-For-Web.pdf.
- International Society for Pharmaceutical Engineering. Good Practice Guide: Practical Implementation of the Lifecycle Approach to Process Validation, March 2019. https://ispe.org/publications/guidance-documents/good-practice-guide-process-validation
- International Conference of Harmonisation. ICH Quality Guideline Q9: Quality Risk Management, 2005. https://database.ich.org/sites/default/files/Q9%20Guideline.pdf.
- Pazhayattil, Ajay Babu, et al. A Semi-Quantitative Risk Assessment Methodology Fit for Bio-Pharmaceutical Lifecycle Stages, PDA J Pharm Sci Tech 75, Issue 6, Feb 2020. https://doi.org/10.5731/pdajpst.2019.010173.
- Pazhayattil, Ajay Babu. “Biopharma Equipment Design, Qualification and Monitoring Model,” Journal of Validation Technology 27, no. 6, Dec 2021.
- International Conference of Harmonisation. Draft ICH Quality Guideline Q9(R1): Quality Risk Management (R1), International Conference of Harmonization. https://database.ich.org/sites/default/files/ICH_Q9-R1_Document_Step2_Guideline_2021_1118.pdf.
- Parenteral Drug Association. Technical Report No. 84: Integrating Data Integrity Requirements into Manufacturing & Packaging Operations. PDA: Bethesda, 2020. https://www.pda.org/bookstore/product-detail/5801-tr-84-data-integrity.



 Ahmed Elsaid, is a Quality Senior Director at Emergent Biosolution Rockville site. Ahmed has over 21 years experience in the pharmaceutical industry as a quality leader in parenteral manufacturing. He has diverse experience working in four different continents addressing the different regulations and managing projects in different phases of the life cycle. His experience spans multiple disciplines including quality systems, aseptic processing, validation, analytical and process development, commissioning, and qualifications.
Ahmed Elsaid, is a Quality Senior Director at Emergent Biosolution Rockville site. Ahmed has over 21 years experience in the pharmaceutical industry as a quality leader in parenteral manufacturing. He has diverse experience working in four different continents addressing the different regulations and managing projects in different phases of the life cycle. His experience spans multiple disciplines including quality systems, aseptic processing, validation, analytical and process development, commissioning, and qualifications. Ajay Babu Pazhayattil, has been providing strategic direction and leadership is his roles with brand, generic, biosimilar, and CDMO organizations. He is currently Vice President, Scientific and Regulatory Affairs at Capcium Inc., Montreal. Ajay is an industrial pharmacist successful in delivering tangible results across segments of pharmaceutical and biopharmaceutical operations. He is a contributing author, speaker, committee lead and influencer in the industry. Ajay has been part of developing and delivering regulatory acceptable product, process remediation strategies.
Ajay Babu Pazhayattil, has been providing strategic direction and leadership is his roles with brand, generic, biosimilar, and CDMO organizations. He is currently Vice President, Scientific and Regulatory Affairs at Capcium Inc., Montreal. Ajay is an industrial pharmacist successful in delivering tangible results across segments of pharmaceutical and biopharmaceutical operations. He is a contributing author, speaker, committee lead and influencer in the industry. Ajay has been part of developing and delivering regulatory acceptable product, process remediation strategies.