B. cepacia: What is it and Why is it a Concern?
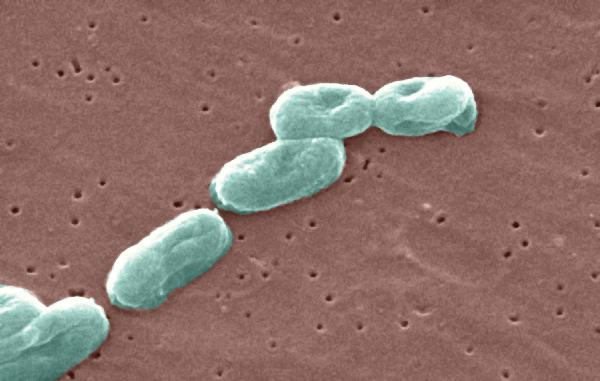
Hospitals and Pharmaceutical Plants on the Prowl for this Uninvited Guest
Burkholderia cepacia has been in the news...again!
Even though this pathogen is not a newcomer (one can easily findpapers from the 1990s discussing its dangers), it continues to wreak havoc in pharmaceutical plants (1). Less than two years ago, two separate batches of oral solid medicine were found to be contaminated by B. cepacia (2,3). The year before,a manufacturer issued a Class I recall for saline flush IV syringes suspected to be responsible for B. cepacia bloodstream infections (4). A quick Web search shows these are not isolated cases. B. cepacia is a major contaminant in both sterile and nonsterile products. A review of U.S. FDA recall data from January 2012 to July 2012found that 39% of contamination cases inn on sterile products were due to the presence of B. cepacia (5).


