Can ICH Q12 Unlock Manufacturing Innovation?
Human medicine has come a long way in the last 100 years. Paradigm-changing therapies have made their way into the clinic since the end of the last century, nurtured by better understanding of the underlying causes of various diseases. These 21st century cures entail personalized therapies using genomics, targeted tumor therapies using antibody–drug conjugates along with cell and gene therapies, just to name a few. These therapies are giving hope to millions of patients. In addition, they profoundly impact multiple stakeholders in the healthcare sector, including the pharmaceutical industry.
Today’s innovative therapies require a complete revision of the traditional manufacturing environment. Manufacturing of the future must become efficient, flexible and agile to adapt to rapidly changing demands and to meet evolving patient needs. Meanwhile, improvements need not compromise the quality and availability of therapies. This implies the use of innovative manufacturing and supply approaches and cutting-edge technologies. And it requires overcoming challenges and barriers to their implementation. In the words of U.S. FDA CDER Director Janet Woodcock, 21st century pharma should be a “maximally agile, flexible manufacturing sector that reliably produces high quality medicines without extensive regulatory oversight.”
The need to revolutionize the technical sector was recognized almost two decades ago, when the first therapies based on recombinant monoclonal antibodies showed huge benefits for patients. Shortly thereafter, work began to develop harmonized guidelines outlining risk- and science-based approaches to product development and manufacturing. ICH Q8–Q11, published between 2005 and 2011, included new, ground-breaking concepts such as quality-by-design, or QbD.
Have we achieved this vision? Only partly. Application of science- and risk-based approaches throughout the product lifecycle have certainly enhanced our product and process understanding. We have also improved robustness of our quality systems and assurance of product quality. Yet we still see little of the regulatory flexibility expected from the new ICH guidelines. Our regulatory filings contain more information and raise more questions than ever before, in part, due to the lack of clarity from regulations during submission. Major challenges consist of variable timelines and submission requirements for post-approval change review and approval. Consequently, globally applicable changes are a logistical challenge that requires excessive time and resources, discouraging innovation. This increases the risk of shortages, supply mistakes and noncompliance situations.
ICH Q12 to the Rescue!
Recognizing the need for action, in 2014, ICH started working on ICH Q12: Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management (Figure 1). The main objectives of ICH Q12 are to facilitate predictability and efficiency of post-approval change management, thus supporting innovation and ensuring sustained product supply. In the ICH Q12 “desired state,” most manufacturing changes will be managed effectively under a company’s pharmaceutical quality system (PQS) without the need for regulatory approval prior to implementation. The extent of such operational flexibility will be subject to the company’s product and process knowledge and confidence in the company’s quality system, which needs to include an effective change management system. This would be supported by effective knowledge and risk management.
ICH Q12 is intended to complement the existing ICH Q8–Q11 guidelines. It includes a core guideline as well as annexes. Key aspects of the Q12 core document cover harmonized tools for established conditions (ECs), planned changes through post-approval change management protocols (PACMPs) and proactive management of the product lifecycle through the product lifecycle management (PLCM) document (see Figure 1). The annexes contain illustrative examples of ECs, PACMPs and PLCM documents.
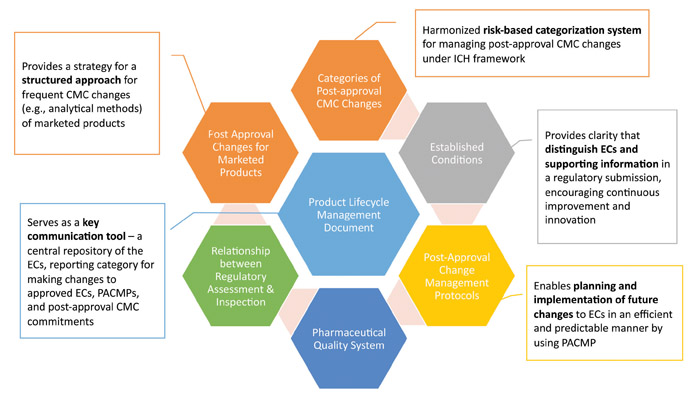
ICH Q12 also considers other important elements to change management. Notably, it explicitly addresses post-approval changes for currently marketed products. Without the ability to apply Q12 approaches to marketed products, the benefits of this guidance would be quite limited. For many organizations, regulatory complexity is the most cumbersome and difficult area to navigate, particularly in a global environment. ICH Q12 describes both a structured approach for frequent CMC changes as well as proposed data requirements. The document also reinforces the criticality of a company’s PQS, particularly as it relates to change management.
This proposed ICH guideline has the potential to be truly transformational, potentially reducing unnecessary cost and time burdens on both the industry and regulators, without compromising high-quality medicines. Perhaps one of the largest gains would be for potential regulatory convergence among ICH regions with respect to defining “regulatory commitments” as opposed to “supportive information” within regulatory submissions. Additionally, implementation of the principles will improve communication and transparency among industry, regulators, reviewers and inspectors. Ultimately, this should lead to greater application of innovative technologies in a timely fashion.
Innovation in manufacturing should be at the heart of our efforts to ensure sustained supply of better, safer medicines to patients. Our industry needs to become faster in adapting the wealth of new manufacturing technologies available to enable product realization, effective control system deployment and continual improvement throughout a product’s lifecycle (ICH Q10: Pharmaceutical Quality System). ICH Q12 will only be a starting point. We have learned from the QbD journey that it will take communication and transparency from both industry and regulators to make the concepts in this guideline a reality that will improve our industry and strengthen our commitment to patients.




