Cross-Industry Insights: Leveraging Quality-Enabling Behaviors for Success
 Drawing a vivid comparison to the tragic crash of United Airlines Flight 173, Jason Kerr, a prominent expert in regulatory intelligence,
compliance and quality culture, delivered a thought-provoking presentation at the PDA/FDA Joint Regulatory Conference 2024 on how the pharmaceutical industry can learn from the aviation sector's approach to quality and safety. Kerr focused on how
fostering a culture of trust, open communication and assertive politeness can transform quality behavior in the pharmaceutical landscape.
Drawing a vivid comparison to the tragic crash of United Airlines Flight 173, Jason Kerr, a prominent expert in regulatory intelligence,
compliance and quality culture, delivered a thought-provoking presentation at the PDA/FDA Joint Regulatory Conference 2024 on how the pharmaceutical industry can learn from the aviation sector's approach to quality and safety. Kerr focused on how
fostering a culture of trust, open communication and assertive politeness can transform quality behavior in the pharmaceutical landscape.
United Flight 173: A Lesson in Communication Breakdown
On December 28, 1978, United Airlines Flight 173, traveling from Denver, Colorado, to Portland, Oregon, ran out of fuel and crashed just outside the Portland airport. The cause was not mechanical failure but a breakdown in communication among the flight crew. While the captain was preoccupied with malfunctioning landing gear, the copilot and flight engineer recognized the urgent need to address the low fuel levels. However, they failed to communicate this assertively, fearing they might undermine the captain's authority. The result was a preventable disaster that claimed 10 lives.
Kerr underscored this event as a prime example of how hierarchical structures and poor communication can lead to catastrophic failures, not just in aviation but also in the pharmaceutical industry. “Quality is about more than just following procedures,” Kerr remarked. “It’s about empowering people to speak up when they see something wrong, no matter their rank or role. So, in our industry, when we say, ‘speak up,’ we really need to!”
Crew Resource Management: A Model for Empowerment
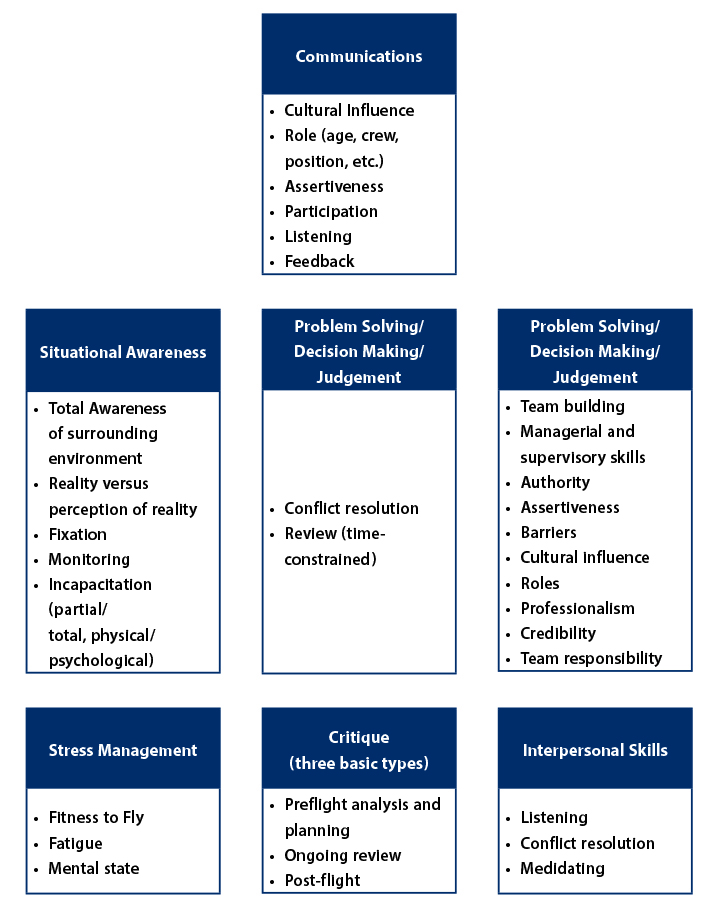
In response to the Flight 173 disaster, the aviation industry adopted Crew Resource Management (CRM), a system designed to flatten hierarchical communication and ensure that all team members—regardless of position—can voice concerns when safety is at stake (see Figure 1). CRM emphasizes assertive politeness, where individuals are encouraged to express concerns firmly but respectfully, ensuring that critical information is not lost in deference to authority.
Kerr highlighted how CRM transformed the aviation industry and significantly improved safety. “Assertive politeness doesn’t mean being rude,” he explained. “It’s about fostering an environment where people feel confident and obligated to voice concerns respectfully but clearly. This can and should be emulated in the pharmaceutical industry.”
Building Trust to Enable Quality Behavior
Kerr emphasized that one of the key components of CRM is trust—trust that every team member, regardless of their role, has the expertise and responsibility to contribute to safety and quality outcomes. He argued that this principle must be carried over to the pharmaceutical sector, where quality lapses often occur because employees are hesitant to challenge decisions or report issues.
"Trust and understanding are fundamental,” Kerr stressed. “Pharmaceutical organizations must create environments where every employee, from manufacturing to quality control, trusts that their input is valued, even when it challenges the status quo."
Parallels to Pharma: Moving Beyond Compliance

Kerr’s message resonated with the conference audience as he drew parallels between aviation and pharmaceuticals. Both industries operate under high-stakes environments where lives are on the line, and failures can have dire consequences. He urged the pharmaceutical sector to move beyond simple regulatory compliance and embrace a culture of continuous quality improvement driven by open communication and trust.
“Just as in aviation, quality failures in our industry often stem from a lack of communication and engagement at critical moments,” he noted. “Implementing systems like CRM can help us foster a culture where quality is everyone's responsibility, and risks are addressed before they escalate.”
Kerr concluded his presentation by emphasizing that the lessons from the aviation industry’s CRM model are directly applicable to pharmaceutical manufacturing. By embracing assertive politeness, fostering trust and ensuring open communication, the industry can build a mature quality culture where quality-enabling behavior becomes second nature.
“We need to ensure that every team member understands their role in safeguarding quality and, more importantly, that they feel empowered to act on it. Quality is not just about compliance; it's about behavior and culture,” Kerr asserted.




