Cryogenic Storage Challenges for Container-Closure Systems

Photo courtesy of SCHOTT.
[Editor’s Note: The study below was jointly conducted by ARaymondLife and SCHOTT. Once more long-term data is available, both authors may consider submitting to a peer-reviewed journal.]
Cell and gene therapy products are typically stored at -180°C and -80°C, respectively, throughout their lifecycle to provide a safe environment to the drug substance and prevent degradation, but such extreme cold temperatures can negatively impact vial-based container-closure systems.
While cryopreservation keeps the product in a stable state, allows for a longer shelf life and ensures stability throughout the supply chain, manufacturers must plan for the unique challenges for vial-based container closure systems.
For example, primary packaging components, such as glass vials, rubber stoppers, and aluminum seals, exhibit contraction during cryogenic storage due to differences in their thermal expansion rates. The contraction of primary components at cryogenic temperatures in conjunction with individual component defect(s) and improper component assembly/compatibility can potentially lead to leakages and/or container closure integrity (CCI) failures.
Typically, in a system where the vial is closed with an aluminum crimp and a rubber stopper, the rubber stopper can deform and maintain the system’s integrity. However, at temperatures below the glass transition of the elastomer (Tg about -50°C), the stopper becomes glassy solid. Then it is crucial to verify if the system retains its integrity (1). In fact, a few cell and gene therapy companies have had to recall their products due to container closure integrity failures, as primary packaging component compatibility was not established (2).
Therefore, there is a need to have a validated container closure system to maintain container closure integrity during storage of these products.
A recent study analyzed a push-fit cap system produced by ARaymondLife combined with rubber stoppers at -80°C to test if this system would fall under USP <1207> Package Integrity Evaluation - Sterile Products. This USP chapter provides guidance in package integrity (leak) testing to ensure that the given package protects the filled sterile pharmaceutical product and meets all physicochemical and microbiological label-claimed specifications through expiry, i.e., sterility preservation, formulation loss preservation, and critical gas headspace preservation. The analysis also fell under USP chapters <1207.1> Package Integrity Testing in the Product Life Cycle - Test Method Selection and Validation and <1207.2> Package Integrity Leak Test Technologies, both of which provide a guideline for container closure integrity test method selection and testing technologies.
Although, several CCIT methods (deterministic and probabilistic) are available for container closure systems as per <1207.1> and <1207.2>, helium leak test and laser-based headspace analysis methods have been routinely used for detection of submicron leaks (3–5).
The study included the following primary packaging components as outlined in Figure 1 as well as the following CCIT instruments and study conditions:
- Glass vials: SCHOTT Fiolax® 2R and 6R ISO designs (hereinafter designated as ISO vials) representing 13 mm and 20 mm crown diameters, respectively.
- Rubber stoppers: Suppliers A and B in 13 mm and 20 mm crown diameters.
- Closure: RayDyLyo® CTO13 & CTO20 (hereinafter designated as plastic push-fit cap) and flip-off aluminum caps with 13 mm and 20 mm diameters.
A headspace oxygen analyzer served as the container closure integrity test instrument. This instrument was calibrated, and performance checked for accuracy (NIST-traceable oxygen standards) and precision (ten consecutive measurements of a sample) at T0 and at each time point. The plastic push-fit cap and the stopper were sterilized using gamma irradiation. Unfilled samples (n = 10 for each configuration) were purged with nitrogen prior to capping with plastic push-fit caps or crimping with aluminum seals. The evolution of oxygen level in the headspace was measured at room temperature for T0 and each subsequent time point of the study (see Figure 2). Incubation was -80 ± 2°C (real time). Time points were six months, one year and two years. Further long-term studies are in the process of being performed for 3 years as well.
 Figure 1 Overview of Primary Packaging Components
Figure 1 Overview of Primary Packaging Components
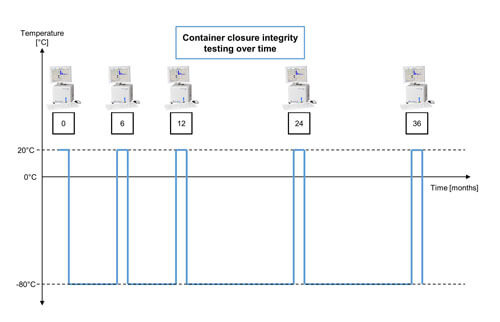 Figure 2 Overview of the Container Closure Integrity Test Protocol Over Time
(The oxygen level in the headspace was measured at room temperature for each time point of the study. After the measurement, the samples are stored at -80°C again.)
Figure 2 Overview of the Container Closure Integrity Test Protocol Over Time
(The oxygen level in the headspace was measured at room temperature for each time point of the study. After the measurement, the samples are stored at -80°C again.)
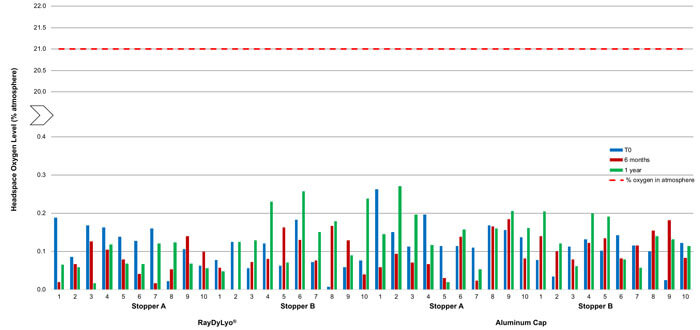 Figure 3 Average headspace oxygen level of CTO13 gamma sterilized samples at various time points after storage at -80 ± 2°C. The red dotted line indicates the maximum oxygen level possible in the atmosphere.
Figure 3 Average headspace oxygen level of CTO13 gamma sterilized samples at various time points after storage at -80 ± 2°C. The red dotted line indicates the maximum oxygen level possible in the atmosphere.
Oxygen ingress data on 2R samples (13 mm) stored at -80°C
As shown in Figure 3, the 13 mm vial system samples, with gamma-sterilized stoppers, stored at -80°C, did not show any significant ingress of oxygen in the headspace within a year of storage, indicating that CCI is maintained during this time period.
The results were:
- Stopper A and a plastic push-fit cap showed an average ingress of 0.12% atm at T0 and 0.08% atm after one year
- Stopper B and a plastic push-fit cap showed an average ingress of 0.08% atm at T0 and 0.15% atm after one year
- Stopper A and an aluminum cap showed an average ingress of 0.15% atm at T0 and 0.15% atm after one year
- Stopper B and aluminum cap showed an average ingress of 0.10% atm at T0 and 0.13% atm after one year
Oxygen ingress data on 6R samples (20 mm) stored at -80°C
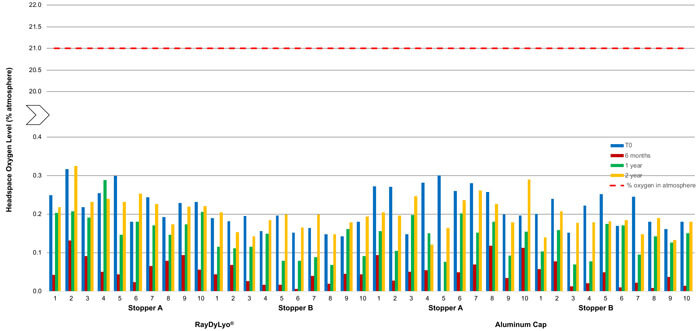 Figure 4 Average headspace oxygen level of CTO20 gamma sterilized samples at various time points after storage at -80 ± 2°C; The red dotted line indicates the maximum oxygen level possible in the atmosphere.
Figure 4 Average headspace oxygen level of CTO20 gamma sterilized samples at various time points after storage at -80 ± 2°C; The red dotted line indicates the maximum oxygen level possible in the atmosphere.
Similarly, the 20 mm vial system samples, with gamma-sterilized stoppers, stored at -80°C, did not show any significant ingress of oxygen in the headspace within two years of storage, indicating that container closure integrity is maintained during this time period (Figure 4).
The results were:
- Stopper A and a plastic push-fit cap showed an average ingress of 0.24% atm at T0, 0.19% atm after one year and 0.23% atm after two years
- Stopper B and a plastic push-fit cap showed an average ingress of 0.17% atm at T0, 0.11% atm after one year and 0.18%atm after two years
- Stopper A and an aluminum cap showed an average ingress of 0.25% atm at T0, 0.15% atm after one year and 0.21% atm after two years
- Stopper B and an aluminum cap showed an average ingress of 0.20% atm at T0, 0.13% atm after one year and 0.17% atm after two years
Conclusions
When evaluating the storage of product at -80°C, it is important to select a suitable container closure system for the secured storage of gene therapy products at cryogenic temperatures. These results of this study suggest that the two types of vials (2R and 6R) in combination with the tested stoppers and plastic push-fit caps (RayDyLyo® CTO13 and CTO20) can safely store product at -80°C. Plastic push-fit caps show reliable results, maintaining container closure integrity for a period of at least one year at -80°C, offering an alternative sealing system to traditional aluminum caps.
Still, manufacturers considering switching from aluminum caps should also evaluate the combination of all the components using the available capping equipment under normal conditions of use.
References
- Iacocca, R. “Primary container design for drug substance and drug substance at Cryo- and cold temperature,” Presented at the 2019 PDA Europe Parenteral Packaging, Venice, Italy, March 19, 2019.
- Duncan, D. “Determining CCI during Deep Cold Storage.” [Webinar] LightHouse Instruments, 2019.
- Kirsch, L. E., et al. “Pharmaceutical container/closure integrity. I: Mass spectrometry-based helium leak rate detection for rubber-stoppered glass vials.” PDA JPST 51 (1997): 187–194
- Sudo, H., et al. “Development of a nondestructive leak testing method utilizing the headspace analyzer for ampoule products containing ethanol-based solutions.” PDA JPST 66 (2012): 434–444.
- Pelaez, S. S., et al. “Comparing Physical Container Closure Integrity Test Methods and Artificial Leak Methodologies.” PDA JPST 73 (2019): 220–234.



 With 30 years of experience in the field of medical devices, in-vitro diagnostic and pharmaceutical packaging, Pascal Sircoulomb has been holding sales, marketing and management positions within large international companies (BD, Danaher/Leica) as well as start-up biotech firms. He joined ARaymondlife in 2018 as Business Development Director
With 30 years of experience in the field of medical devices, in-vitro diagnostic and pharmaceutical packaging, Pascal Sircoulomb has been holding sales, marketing and management positions within large international companies (BD, Danaher/Leica) as well as start-up biotech firms. He joined ARaymondlife in 2018 as Business Development Director Luce Sohier holds a five-year degree diploma in biochemistry and microbiology from the University of Nancy, France. She has worked in upstream processing in a collaboration with Merck Millipore and Pall, and downstream processing with West and SCHOTT. Currently she is in charge of business development for sterile solutions at SCHOTT in Europe.
Luce Sohier holds a five-year degree diploma in biochemistry and microbiology from the University of Nancy, France. She has worked in upstream processing in a collaboration with Merck Millipore and Pall, and downstream processing with West and SCHOTT. Currently she is in charge of business development for sterile solutions at SCHOTT in Europe.