Glass Along the Value Chain Certain Treatments and Their Impact on Extractables for Borosilicate and Aluminosilicate Glass
Protecting medications from harmful environmental influences and preserving their efficacy during shelf life are two of the most pressing challenges for both packaging manufacturers and pharmaceutical companies.
In particular, packaging materials must adhere to stringent regulations. U.S. regulations state that: “Equipment shall be constructed so that surfaces that contact components, in-process materials, or drug products shall not be reactive, additive, or absorptive so as to alter the safety, identity, strength, quality, or purity of the drug product beyond the official or other established requirements” (1). This is also reflected in EU regulations (2).
For this reason, high-quality borosilicate glass containers are currently the preferred primary packaging material, even for the most sensitive drugs. But as new glass compositions enter the market, how do these measure up? The case study below seeks to answer this question.
According to the current U.S. and European pharmacopeias, borosilicate glass contains significant amounts of boric acid, aluminum oxide, alkali metal oxides and alkaline earth metal oxides. Furthermore, due to the chemical composition of the borosilicate glass, it bears high hydrolytic resistance and, therefore, is classified as a Type I glass container (3). The resistance against water attack is assessed according to two test methods: the glass grains test and the inner surface test. Both of these determine the sodium extraction (including calcium and potassium expressed as sodium oxide equivalents) of glass after a certain stress procedure (autoclaving).
The result of the glass grains test directly depends on the composition of the glass, and—provided the composition is not changed—remains constant. On the other hand, the hydrolytic resistance of the inner surface tends to be negatively affected during the transformation of a glass tube into a container (converting).
A Salty Comparison Study
Whereas the root cause for resistance, and all other influencing factors along the value chain, are well known and have been intensely studied for borosilicate glasses, information is lacking for other glass types, such as aluminosilicate glasses. Consequently, a comparative study of aluminosilicate and borosilicate glass was performed for each step of the value chain with regard to the influence of converting and post-treatments on the extractables level for each glass type. This study specifically focuses on the conversion and ion-exchange process.
Here, the amount of network modifiers (sodium, calcium, magnesium, potassium) extracted from the inner surface of the glass tubing is compared to the respective amount extracted from the vial (i.e., after converting of the tubing). Since a glass might require an ion-exchange process after converting, a surface test is also performed on the vials following this chemical treatment (Figure 1).

The tubing sections, as well as the vials (nominal volume: 2R), are filled with ultrapure water and autoclaved for one hour at 121 °C per ISO 4802-2 (4). The analysis of the extracted elements is performed by means of ICP-MS and ICP-OES. Since the surface area exposed to the water is different for tube sections (the end is closed by a stopper, so a smaller glass surface comes in contact with water) and vials (due to a glass bottom, more glass surface comes in contact with water), the results are given in μg/cm2. This is a surface-correlated value, allowing for an exact correlation between tubing sections and vials. Figure 2 shows the results obtained, given as oxides. Right from the start, namely for the glass tubing, the amount of extractables is shown to be more than 400% higher for aluminosilicate glass compared to borosilicate glass (sum of network modifiers: 0.99 μg/cm2 vs. 0.24 μg/cm2), as has also been reported in a previous case study published in the PDA Letter (5).
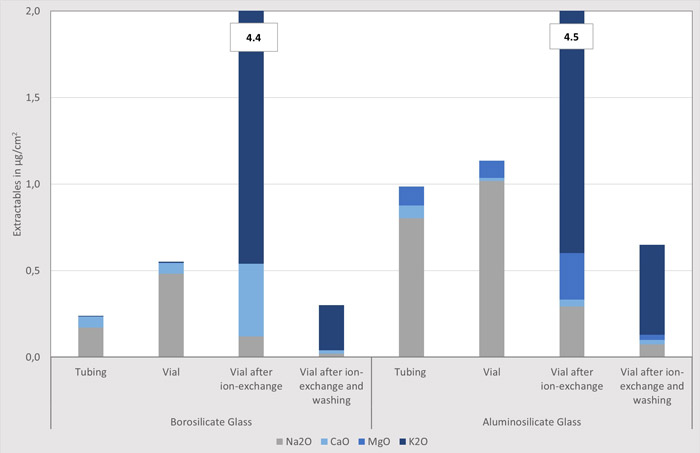
Figure 2 also shows that, for both glass types, the levels of extractables increase after conversion of the glass tube into a vial. For borosilicate glass, this is mainly due to evaporation of volatile components such as alkali borates. This phenomenon, including all possible influencing factors, has been heavily studied over the last few decades and, thus, is well understood and clearly under control.
Also for the aluminosilicate glass type a negative, albeit, smaller influence of the converting step is demonstrated. Since there is no boron present in the aluminosilicate glass type, however, an alkali borate evaporation cannot explain the increase of sodium extraction after forming of the aluminosilicate glass vial. In consequence, the root cause of this increase remains unclear and needs to be investigated
Going one step further, the greatest increase can be seen for a glass vial after the ion-exchange process. During this post-treatment, also referred to as chemical strengthening, the container is dipped into a liquid salt bath, exchanging sodium ions from the near- surface region of the glass with potassium ions from the salt bath. Since both glass types contain significant amounts of sodium this treatment can likewise be applied for aluminosilicate glass as well as borosilicate glass. The effect on the extractables level is as fol- lows. As expected, the amount of sodium decreases, yet potassium dominates the level of extractables. Here, it amounts to 3.9 μg/ cm2 for borosilicate (total 4.4 μg/cm2) as well as for aluminosilicate glass (total 4.5 μg/cm2). Thus, the potassium extraction of a glass vial after the ion-exchange process (3.9 μg/cm2) is found to be more than six times higher than the combined extraction level of all network modifiers (sodium, calcium, potassium, magnesium) of an untreated borosilicate glass vial (sum: 0.6 μg/cm2). This might be due to residues of the potassium salt (e.g., potas sium nitrate) on the inner surface of the glass, although the procedure laid down in ISO 4802-2 (subchapter 8.2) already includes a cleaning procedure of rinsing with water at least five times (4).
With an appropriate washing step, it is possible to remove residues of a potassium salt layer so that the amount of extractables is significantly reduced. As expected, the extraction profile of the network modifiers is still dominated by potassium oxide. Keep in mind, the level is even lower compared to the untreated vial: 0.30 μg/cm2 versus 0.55 μg/cm2 for the borosilicate glass and 0.65 μg/cm2 versus 1.14 μg/cm2 for the aluminosilicate glass. This is an absolute prerequisite for the aluminosilicate glass, since the post-treatment not only needs to eliminate the negative effect of the ion-exchange process but is also indispensable for reducing the high level of extractables of the untreated aluminosilicate glass.
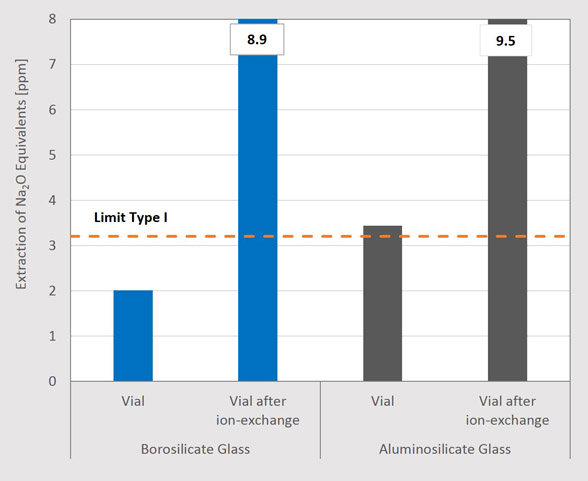
With respect to the regulatory requirements, generally speaking, a higher amount of extractables means a lower chemical and hydrolytic resistance. To determine the compliance with the Type I requirements, the results of this case study are expressed as sodium oxide equivalents (Na2O, CaO, K2O) following the regulatory prescriptions of ISO 4802-2 and the European Pharmacopoeia, respectively (see Figure 3) (3, 4).
Figure 3 highlights that a 2R borosilicate glass vial without any treatment is, as expected, well below the Type I limit. Yet, the untreated vial made of aluminosilicate glass exceeds the Type I limit and, therefore, would only meet Type III requirements. After the ion-exchange process, the amount of Na2O equivalents is roughly three times the limit value of a Type I glass for both glass types (8.9 ppm for borosilicate glass, 9.5 ppm for aluminosilicate glass). The negative influence of this treatment on the extractables level is apparent. The study indicates that glass vials cannot be used for packaging directly after a strengthening/ion-exchange process, and, as a consequence, further treatment or leaching process must be applied in order to fulfill regulatory requirements.
In summary, the amount of extractables depends on the glass type, yet also changes significantly throughout the value chain increasing the risk of inaccurate comparisons. Starting from the initial level of the tubing, the negative influence of the converting and ion-exchange process on the extractables profile of both glass types, borosilicate as well as aluminosilicate glass, is shown. Compared to borosilicate glass vials, aluminosilicate glass vials after converting, i.e., before the ion-exchange process, show a high amount of extracted elements that makes untreated aluminosilicate glass containers unfit as parenteral primary packaging material. As a consequence, further treatments or leaching processes should be applied on aluminosilicate glass containers before they may come into contact with the drug.
References
- Guidance for Industry: Sterile Drug Products Produced by Aseptic Manufacturing–Current Good Manufacturing Practice, U.S. Food and Drug Administration, September 2004. www.fda.gov/downloads/Drugs/Guidances/ucm070342.pdf
- European Commission. EU Guidelines to Good Manufacturing Practices. In EudraLex: Volume 4, 2010.
- Council of Europe. Chapter 3.2.1 Glass containers for pharmaceutical use. In European Pharmacopoeia, 8th ed., 2017.
- International Organization for Standardization. ISO 4802-2:2016 Glass-ware–Hydrolytic resistance of the interior surfaces of glass containers—Part 2: Determination by flame spectrometry and classification, 2016. https://www.iso.org/standard/67783.html
- Heinl, C. Extractables Profile of Aluminosilicate Glass Prior to Chemical Treatments. PDA Letter (2017) 53:23.



 Claudia Heinl is a Product Manager for SCHOTT Pharmaceutical Tubing.
Claudia Heinl is a Product Manager for SCHOTT Pharmaceutical Tubing.