Glass Breakage in Pharmaceutical Packaging Highly Welcome or Utmost Feared?
.jpg?sfvrsn=8417898e_0)
Thanks to low extractable/leachable profiles, small diffusion coefficients and high transparency, glass reigns as the undisputed material of choice for parenteral packaging. For a long time, the only major drawback of glass was believed to be its inherent breakage risk. Yet recent concerns about breakage have prompted some parenteral manufacturers to take another look.
Thanks to low extractable/leachable profiles, small diffusion coefficients and high transparency, glass reigns as the undisputed material of choice for parenteral packaging. For a long time, the only major drawback of glass was believed to be its inherent breakage risk. Yet recent concerns about breakage have prompted some parenteral manufacturers to take another look.
Regarding both of these areas of interest, three questions should be addressed:
- What is the right level of container strength and how can it be achieved?
- At which step of the value chain should the strength and the integrity of the container be investigated?
- What is the appropriate test scenario regarding container strength and integrity to make container performance predictable?
To answer the first question, a short introduction to glass strength is required. In general, the probability of breakage or the mechanical strength of glass, respectively, is dependent on the existence of surface flaws and the magnitude of applied tensile stress rather than on glass composites (1). Based on theoretical formulas, like the Griffith equation, one can derive two general rules of thumb: 1) the bigger the surface flaws, the less tensile stress can be applied until glass breakage occurs, and 2) glass products with only very small defects can withstand much larger tensile stress before fracturing occurs. Moreover, since no container is fully alike with respect to surface quality, glass breakage can never be exactly predicted.
Based on the tremendous negative impact of surface flaws on mechanical stability of the final product, two approaches are commonly used to control the mechanical strength of glass: (i) minimizing the generation of surface flaws along the full value chain, e.g., via fill/finish line optimization or special glass coatings at the outside of the container, and (ii) lowering the destructive impact of existing surface flaws via post-processing, e.g., via chemical toughening. Within process (ii), intended built-in stress profiles are generated through systematic ion exchange.
The good news is that there is no need for a special glass type for either approach. All common silicate glasses, such as sodalime, aluminosilicate and borosilicate glass, are suitable. For approach (i) there is no restriction. The bad news is that manipulations of the mechanical strength via chemical toughening not only affect breakage resistance, but may also influence fracturing behavior, glass chemistry of the inner surface and, the extractable/leachable profile of the pharmaceutical container (2). The last aspect has to be taken into account when it comes to regulatory container approvals and drug shelf life-studies, particularly for drugs already on the market, leading to significant costs due to repetition of shelf-life studies along with other regulatory requirements. Since chemical toughening requires additional post-processing steps, increased purchasing costs for the container itself and additional manufacturing costs must be considered, too. For variations in fracturing behavior, it is the crack formation risk, or CCI and fill/finish line performance that needs to be reinvestigated.
To Chemically Strengthen or Not
To demonstrate possible ambiguous fracturing behaviors, a study compared chemically strengthened and nonstrengthened containers. Both types were either clamped between two metal plates while the mechanical load was continuously increased along the vertical axis until breakage occurred, or sawn with a diamond plate to depict the container’s fracturing behavior upon crack formation. A chemically strengthened container can withstand high mechanical load if clamped between two metal plates and bursts apart into predominantly superfine particles upon breakage. Conversely, if the same type of container is scratched by a sharp, very stiff material, it suddenly breaks apart into rather large cullets. In contrast, nonstrengthened glass containers withstand less mechanical load if clamped between two metal plates, resulting in much larger fragment sizes compared to a strengthened container. When in contact with a sharp, very stiff material, cracks do not necessarily lead to breakage. Here, even deep furrows can be easily sawn into such containers without full destruction.
The differences in fracturing characteristics between both types of containers can cause different scenarios during container use, such as on filling machines. The first assumption, that strengthened containers allow a higher production yield due to less glass breakage proved incorrect. Since strengthened glass can show even higher mechanical strength than certain machine parts, not only the breakage of glass needs to be considered, but also the destruction of machine components due to too-strongly clamped containers. Thus, an increase of Total Cost of Ownership (TCO) might result, due to longer machine downtimes and a reduction in machine throughput. Taking this into account, an outer coating of nonstrengthened containers that minimizes glass-toglass contact might work better if aiming for a good production yield.
In theory, but still without statistical evidence, strengthened containers might lower the risk of jeopardizing patients’ health through imperceptible ingress of impurities into the aseptic drug, since crack formation on strengthened containers results in immediate breakage.
Accordingly, concerns about the correct container strength cannot be sufficiently addressed as it is rather a question of defining the perfect match between container properties and optimum processing. Here, the supply chain must be factored in as well. How small the risk of a crack or breakage actually is can be exemplified by looking at some statistics; projections estimate 20 billion vials for filling injectable drugs are processed on an annual basis, while only six recalls related to “cracks” and “breakage” were announced within the last six years for borosilicate glass containers (3). Already starting from a very low-risk potential, the primary goal is to lower it even further in order to finally achieve “zero defects.” Thus, what happens if crack formation becomes fully negligible in the future through line optimization? Plus, what if cracks remain undetected?
Three-Step Process for Analysis
This brings us directly to the next question: “At which step of the value chain should the strength and the crack formation risk of the container be investigated?” In general, if the glass surface is not regenerated via additional processing steps, like fire polishing or etching with harsh chemicals, products made of glass will have a nonerasable memory regarding surface defects. In other words, surface flaws on the container accumulate throughout the entire process chain and continuously lead to a reduction of container strength. Considering the entire value chain, from glass melt all the way to the end user, it is obvious that there cannot be one single test scenario for glass breakage or crack formation risk, respectively, that models all the numerous processing steps.
Similar to the recommendation within USP <1207> Sterile Product Packaging—Integrity Evaluation, three test stations along the full value chain seem to be reasonable: (Station a) right before, (Station b) during and (Station c) after containers pass through the fill/finish line (Figure 1). Of course, if shelf-life stability tests are included, additional studies have to be conducted, too. Conversely, investigations that are supposed to characterize the mechanical stability of glass tubing or freshly converted vials (no coatings, no chemically strengthening, etc.) are rather less informative. For both product categories, there is still a long way to go before the product reaches the end user. Here, it is much more important to focus on the intactness of the glass by detecting any existing surface flaws.
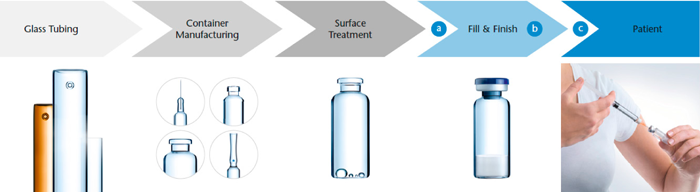
Tests immediately before containers enter the fill/finish line (Station a) are relevant not only for sorting predamaged containers but also for differentiating container categories, like strengthened containers, nonstrengthened containers, bulk containers and ready-to-use containers. Next, in-line monitoring of containers passing through the fill and finish line (Station b) can help identify high-risk areas causing surface flaws on the container. Investigating vials directly after the fill and finish line with respect to mechanical stability and leakage due to crack formation (Station c) are the most relevant for patient safety since this reflects end-user container stability the most. Since each test station answers a different question of container strength and CCI, each station must be equipped with a different experimental setup.
Table 1 summarizes potential experimental methods that can be applied at the three given stations. Moreover, it clarifies whether the measurement is destructive or nondestructive. Taking into account that container strength tests only allow statistical statements due to the destructive nature of such tests, there can never be a 100% guarantee in terms of container strength. In contrast, tests intending to detect cracks can be both destructive and nondestructive. Cracks are either detectable directly via camera inspection units or indirectly via CCI analysis methods.
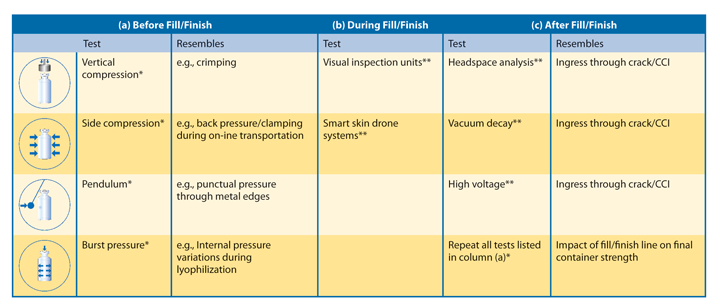
As previously mentioned, tests in Station a mainly focus on the investigation of mechanical strength to reflect progress in product development of new container systems. Since mechanical strength cannot be directly implemented into the filling line due to its destructive nature, lab tests need to be developed that try to resemble the numerous mechanical loads to which a container is exposed on a fill/finish line. Possible test scenarios could be vertical compression (e.g., resembling the crimping process), side compression (e.g., resembling back pressure during container transport in depyrogenation tunnel), pendulum (e.g., resembling punctual impact through metal edges) and burst pressure (e.g., resembling pressure differences within a closed container during lyophilization). All respective test scenarios are depicted in Table 1. After completing the various mechanical tests, one should not stop with the investigations. Here, subsequent fractographic analysis provides a promising tool to identify the origin of the breakage and possibly ascertain the weakness of the analyzed container (4).
Conducting not just one mechanical stability experiment, but several different tests is very important. The latest in-house studies indicate that each type of container (other glass type, other surface treatment, other converter, etc.) has its own fingerprint when it comes to the correlation between the different mechanical strength tests. For instance, by conducting burst pressure stability tests, the outcome for axial compression is not predictable.
Nowadays, visual camera inspection systems are well established on fill/finish lines for Station b and can even be considered as a standard feature. New technologies, however, have evolved explosively over the last few years. For example, mathematical algorithms based on neural network programming might soon facilitate fast learning and adaptive online inspection units for even small lot sizes with fast changing container dimensions.
On top of this, another new technology has launched recently. With this, a drone container passes through the complete fill/finish line together with regular glass containers, detecting pressure, spin, tilting and shock. With this approach, optimizing production lines with respect to any potential mechanical stress that may cause surface flaw generation, and thus CCI issues, is now possible. Once identified, high-risk areas within production are often easy to eliminate. Since smoothly running lines accompany low production losses, this new method may reduce TCO, too (5).
Coming to Station c—the most crucial indicator for ensuring patients’ health—nondestructive test methods to ensure API policy for CCI investigations should be indispensable for future pharmaceutical packaging. So far, however, regulators only prescribe “100% integrity testing” for fused containers like glass ampoules (6). For other types of containers (syringes, vials or cartridges), such tests remain only a recommendation (7). Nonetheless, on-line, fully integrated CCI inspection units like high-voltage leak-detection modules, vacuum and pressure decay technologies are already available and well established on the market. Machine outputs of up to 600 containers/min are becoming more common. Hence, there is no longer a limitation regarding the technical feasibility of 100% online inspection systems. Despite CCI evaluation, the mechanical strength of the container also has to be examined. By repeating the analysis described for Station a, potential weaknesses in the fill/finish line might be identified. Moreover, only at this stage can the “real” mechanical stability of a used container be evaluated.
To conclude, all types of containers have their own specific advantages or disadvantages regarding crack formation risk and processability. Still, not all the solutions outlined will enable full control over the inherent nature of glass breakage. Yet well-positioned measurement setups in combination with new technology can help to make it more assessable. [Editor’s Note: The online version of this article includes additional figures/tables.]
References
- Shelby, J.E. Introduction to Glass Science and Technology. 2nd Ed. London: RSC 2005.
- Heinl, C. “Glass along the Value Chain.” PDA Letter 54 (2018): 20–23, 35.
- U.S. FDA Recall Information Search 06/20/2018
- Haines, D. et al. “Why Do Pharmaceutical Glass Containers Break?” IPI 8 (2016)
- Eberle, L., et al. “Data-driven tiered procedure for enhancing yield in drug product manufacturing.” Computers & Chemical Engineering 87 (2016) 82–94.
- EU GMP Annex 1
- USP <1207>



