HEPA Filter Management—Industry Survey Results
In the fourth quarter of 2024, the Parental Drug Association (PDA) undertook a comprehensive survey regarding the management of high efficiency particulate air (HEPA) filters within the pharmaceutical industry.
The survey was created to benchmark industry practices. The objective was to collect information on topics such as filter-recertification testing procedures, recertification failure investigations, the handling and disposal of HEPA filters and data-management practices supporting filter lifecycle. The knowledge gained from obtaining industry practices and comparing it to internal practices will add context to future compliance and process-improvement initiatives. Furthermore, this provides more data on key topics impacting operations to personnel seeking to learn industry practices.
Industry HEPA Filters
To prepare air entering cleanrooms, HEPA filters trap and remove airborne particles such as dirt, pollen, viruses and bacteria to maintain cleanroom air-quality specifications and prevent airborne contamination. HEPA filters serve as a boundary between upstream air handler units and environmentally controlled cleanrooms and can be used upstream as prefilters to reduce the load on downstream boundary filters. HEPA filters are utilized in cleanrooms, process equipment (e.g., dry heat ovens), laminar flow hoods, biosafety cabinets and isolator systems.
HEPA filters are classified as group H according to ISO 29463-1: 2024 High efficiency filter and filter media for removing particles in air, Part: 1 Classification, performance, testing and marking. The ability to capture small particles makes HEPA filters suitable for use in protecting industry cleanrooms from airborne particle contamination. That same ability results in HEPA filters having higher pressure differentials than air filters used for air preparation in office settings.
HEPA Filter Management
The lifecycle of HEPA filters is managed from arrival to disposal, encompassing handling, storage, installation, initial certification, recertification, removal, quality investigations and discard. Recertification testing is conducted at defined intervals to ensure filter integrity. If a filter fails recertification, it is replaced by a certified filter before operations resume.
Filter recertification and replacement can be managed in three ways:
- Run to Failure: Filters are replaced after failing certification tests or observed damage, without or with minimal performance monitoring.
- Conditional Monitoring: Using data gathered from cleanroom and air-handler monitoring, filter-performance tracking and certification-testing to identify failure trends to proactively replace potential failures.
- Time-Based Replacement: In service, filters are replaced proactively after a predetermined duration (based on estimated probable failure) to mitigate risks associated from seal failures and issues causing multiple failures.
Understanding contamination risks in relation to industry standards for handling, replacement and data usage is crucial. Relevant ISO documents detail filter classification, testing methods and expected outcomes:
- ISO 14644-3:2019 Cleanrooms and Associated Controlled Environments, Part 3: Test Methods
- ISO 29463-1:2024 High-Efficiency Filters and Filter Media for Removing Particles in Air, Part 1: Classification, Performance, Testing, and Marking
- ISO 29463-5:2022 High-Efficiency Filters and Filter Media for Removing Particles in Air, Part 5: Test Method for Filter Elements
Upon identifying a HEPA filter for replacement through failed recertification testing, an investigation to identify root cause is performed. The level of rigor of the investigation coincides with the level of risk associated with failure and impact to operation. Thus, high-risk operations in Grade A areas would be treated with more scrutiny and investigation rigor than lower grades. More steps may be warranted such as filing a health authority notification, as applicable, when filter failures are identified.
Understanding the interpretation of regulatory requirements and identifying industry practices in these situations can clarify how to appropriately respond.
The Survey
Ten questions (see Figure 1) were prepared and submitted to PDA to include in the survey. Each question contained multiple-choice answers. Some questions contained an “Other” answer choice that allowed for manual entry of answers not listed. In each case, the “Other” selections were reviewed, analyzed and recategorized in the data analysis.
Sixty-five industry organizations responded to the HEPA Filter Management survey. Each responder represented a separate organization where the identification of the participant remained anonymous. In addition, the identities of the participating organizations were not disclosed to any organization outside the PDA. This approach encouraged participation in the benchmarking process.

Results
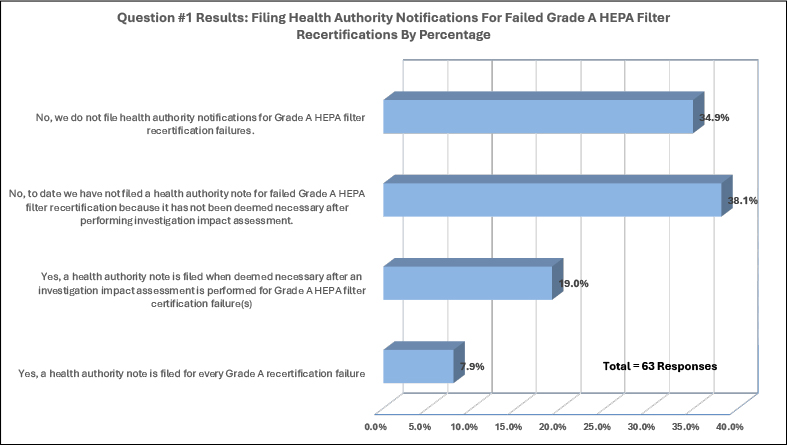
The response to question one regarding health-authority notifications for Grade A HEPA recertification failure highlighted that most companies do not file or have not filed a health-authority notification after performing an investigation (see Figure 2). Out of sixty-three organizations that responded to the first question, seventy-three percent (73.0%) responded “No” by selecting “No, we do not file health authority notifications for Grade A HEPA filter recertification” or “No, to date we do not file health a health authority notification for failed Grade A HEPA filter recertification because it has not been deemed necessary after preforming investigation impact assessment.”
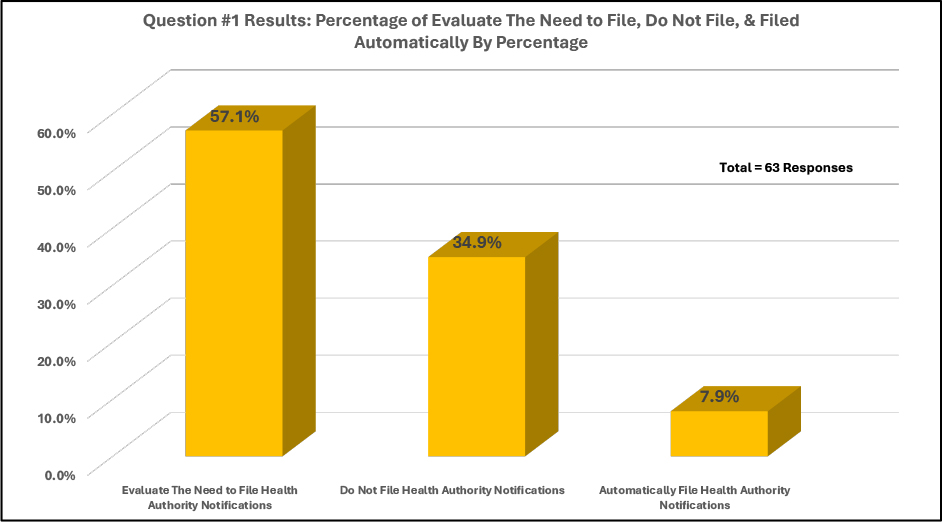
In the second graph of Figure 2, most companies (57.1%) that responded to the survey evaluate the need to file a health-authority notification. A smaller but significant number of survey responders (34.9%) do not file notifications, and only a small percentage (7.9%) file a notification for every certification failure.
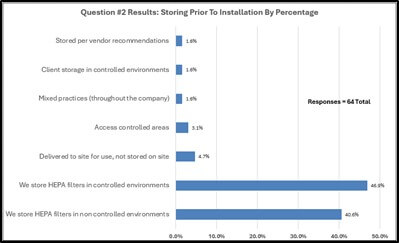
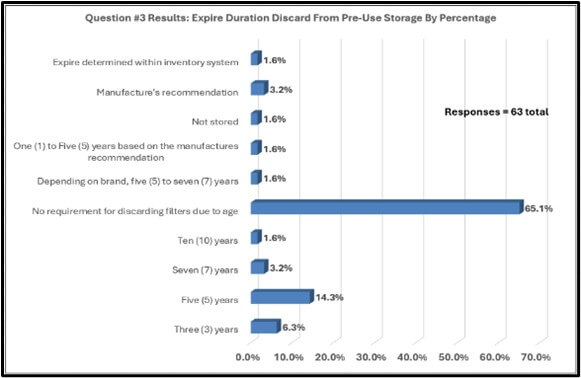
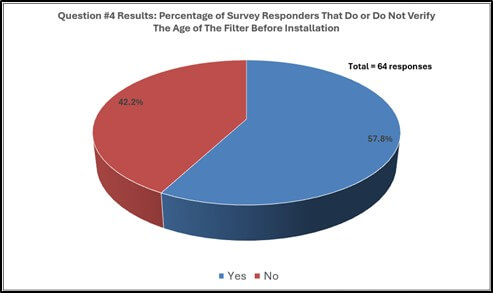
Survey responders gave feedback regarding handling HEPA filters prior to installation (see graphical results in Figure 3). Approximately forty-seven percent (46.9%) of survey responders store filters in controlled environments before use in operations. Sixty-five percent (65.1%) of responders have no requirement for discarding filters due to age prior to filter installation, roughly fifty-seven percent (57.1%) of the sixty-four responders answered “Yes” to verifying the age of the HEPA filter before installation.
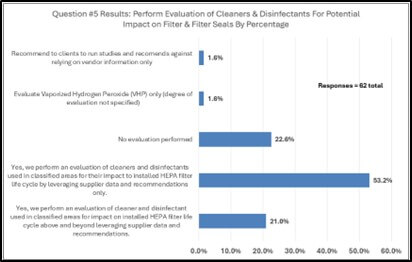
Of the sixty-two survey responders who answered question number five (see Figure 4), fifty-three percent (53.2%) answered that an evaluation of cleaners and disinfectants used in classified areas are evaluated for their impact to the installed HEPA-filter lifecycle by leveraging supplier data and recommendations. A smaller percentage of roughly twenty-three percent (22.6%) do not evaluate the impact of cleaners and disinfectants to HEPA filters while a smaller percentage of approximately twenty-one percent (21.0%) go above and beyond supplier recommendations and perform an evaluation internally.
Question number six followed up question number five and was available to answer based on the response to question number five. A majority of the forty-six responders, fifty-two percent (52.0%), answered that the evaluation of cleaners and disinfectants for use in operations influences the planned service life of the installed HEPA filer in Grade A and B areas.

The strategy for the replacement of HEPA filters and monitoring methods in Grade A and B areas of a sterile facility were the focus of questions seven and eight (see Figure 5). Forty-nine percent (49.2%) of the sixty-three responders to question number seven answered that a combination of conditional monitoring and time-based replacement are used to determine the in-service life of a filter. Responses also indicated that nineteen percent (19.0%) replace filters after failed certification results.
“Alert” vs. “Action” leak-test certification results (48.8%) were selected as the most frequent monitoring method, followed by measuring differential pressure across the filter (39.0%) in question number eight (see Figure 5). A combination of Alert vs. Action leak-test certification results and measuring differential pressure across the filter was selected by seven percent (7.3%) of the survey responders.
.jpg?sfvrsn=e3d9aff_1)
.jpg?sfvrsn=af0bf231_1)
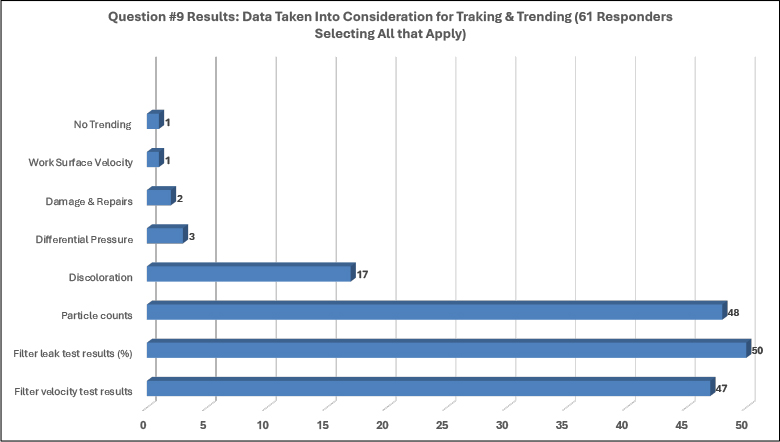
Of the sixty-one responders to question number nine (see Figure 6), eighty-two percent (82.0%) selected filter leak-test results, roughly seventy-nine percent (78.7%) selected particle counts, seventy-seven percent (77.0%) selected filter velocity-test results and approximately twenty-eight percent selected discoloration (27.9%) as data taken into consideration for tracking and trending (Figure 6).
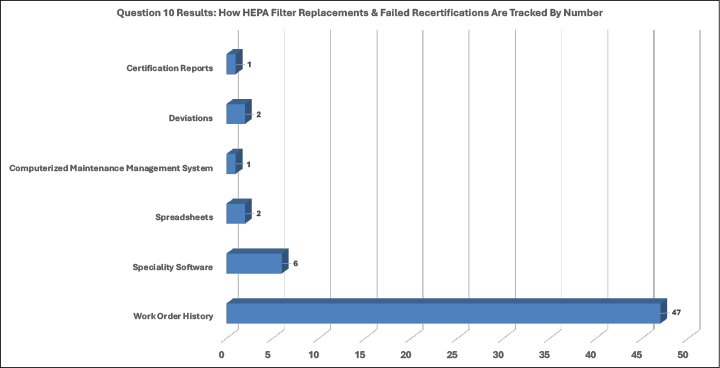
Failed recertifications are tracked by work-order history. Of the sixty-one survey responders who answered question number ten, seventy-seven percent (77.0%) responded that work-order history is used. Just under ten percent (9.8%) listed that they use specialty software to aid in tracking recertification failures and filter replacement.
Conclusion
Industry responders provided valuable insight to how HEPA filters are managed, recertified and tracked in industry cleanrooms. The response data obtained enabled greater understanding of industry practices. Some notable findings include:
- Health-Authority Notifications: Filing a health-authority notification for every Grade A filter failure is not a common practice (7.9%) among sixty-three responses. A large majority (73.0%) of responders do not or have not ever filed a notification for Grade A recertification failures. A majority of the responders (57.1%) evaluate the need to file health-authority notifications with every investigation.
- Filter Discarding Policies: A significant majority of respondents (57.8%) do not have a requirement to discard filters based on age prior to their use in operations.
- Evaluating the Impact of Cleaners and Disinfectants: A majority of sixty-two survey responders (53.2%) perform an evaluation of cleaners and disinfectants used in classified areas for their impact to the installed HEPA-filter lifecycle by leveraging supplier data and recommendations only.
- Monitoring Practices: More survey responders (49.2%) employ a combination of conditional monitoring and scheduled time-based replacement for managing filter replacement than any other method such as “Run to Failure” (19.0%), conditional monitoring only (14.3%) or time-replacement only (14.3%).
- Tracking and Trending: Survey participants take into consideration filter leak-test results (82.0%), particle counts (78.7%) and filter velocity-test results (77.0%) to monitor filter performance and identify trends.
- Recertification Tracking: Most organizations (77.0%) utilize work-order history to track recertification failures.
After reviewing and interpretating the data, organizations can evaluate their internal practices against industry practices. Through survey response evaluations, organizations can identify and target opportunities for compliance and efficiency improvement.
In conclusion, the PDA survey capabilities provide an excellent platform for benchmarking. The PDA personnel managing the survey process kept survey participants anonymous and limited one response per organization to prevent redundant answers. In all, the survey process implemented by PDA personnel encouraged participation. The sixty-five PDA member organizations completing the survey serves as evidence of robust industry engagement, which will encourage the future use of this forum for industry benchmarking activities.



