Highlights of the SBIA Generic Drugs Forum
The Current State of Generic Drugs, this year’s Generic Drugs Forum sponsored by the U.S. FDA Center for Drug Evaluation and Research (CDER) Small Business & Industry Assistance (SBIA), was held virtually April 26-27. Presentations highlighted the data collection process, how integrated risk assessments for drug substance, integrated manufacturing, facilities and unit operations are conducted, and lifecycle integration.
Representatives from CDER’s Office of Pharmaceutical Quality (OPQ)—Peter Capella, PhD, Director Division of Immediate and Modified Release Drug Products, Mayra Pineiro Sanchez, PhD, Senior Pharmaceutical Quality Assessor, Kimberly Raines, PhD, Branch Chief, Division of Biopharmaceutics, and Rakhi Shah, PhD, Associate Director, Office of Pharmaceutical Manufacturing Assessment (OPMA)—provided very insightful overviews of the Knowledge-Aided Assessment and Structured Application (KASA) program and its key benefits:
- Provides a structured approach to drug product assessment that fully supports the decision on ANDA product quality
- Allows a collaborative, multidisciplinary view of quality assessments to ensure a consistent decision-making approach
- Gives FDA internal customers the ability to review the drug product lifecycle using analytics of critical quality attributes, stability, and risk evaluations
Though not stated explicitly, a version of the KASA program will most likely be used for assessment of NDA and biologic applications.
A number of the Forum presentations focused on data integrity, quality culture and senior management responsibility, which are the basis for effective drug product quality assessments used for application approval. Shujun Chen, PhD, OPMA Senior Pharmaceutical Quality Assessor, discussed how data integrity (DI) issues may be identified during surveillance inspections, pre-approval inspections, or in submitted application data. She also explained the concurrent and parallel DI assessments between distributed commercial products and pending applications used by internal offices.
Presentations by OPMA Branch Chief, Haitao Li, PhD, and Staff Fellow, Alexander Gontcharov, PhD, focused on how remote interactive evaluations (RIE) and records under 704(a)(4) requests are used as alternate tools for risk mitigation. FDA made it clear that they will initiate all instances of an RIE; they will not accept requests from applicants or facilities to perform an RIE. Further, they indicated that declining a RIE request may delay a regulatory action. FDA is still evaluating the effectiveness of alternative tools and will inform the industry if it will continue the program or make changes.
Note: Section 704(a)(4) of the FD&C Act presents provisions for a remote interactive evaluation or a request for records or other information for facilities.
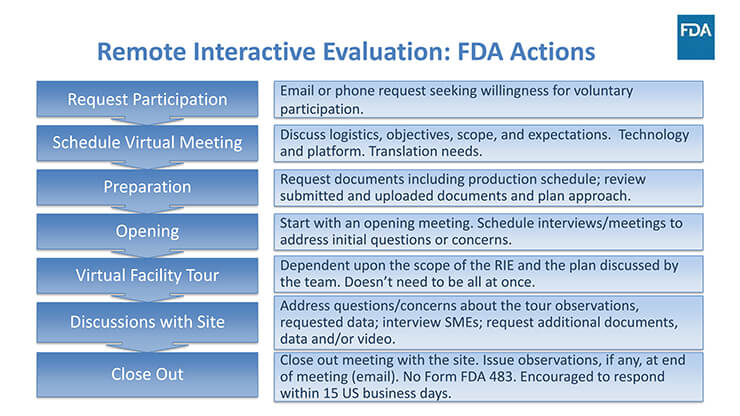 Figure 1 FDA’s actions with respect to Remote Interactive Evaluations
Figure 1 FDA’s actions with respect to Remote Interactive Evaluations
Two OPQ presentations on the evaluation of a firm’s pharmaceutical quality system (based on ICH Q10) and the use of ICH Q12 for product lifecycle management were especially interesting.
Alex Viehmann, Division Director, Division of Quality Intelligence II, presented on internal knowledge management programs of the Office of Quality Surveillance and how they are used. Both qualitative and quantitative assessments of a facility are employed to determine the effectiveness of a site’s quality system and, using machine learning and predictive analytics, to make predictions for their effectiveness metrics. The goals are to predict early on a site’s future state, share assessments with field investigators, and support decisions regarding original submissions and supplements.
Ashley Boam, Director, Office of Policy for Pharmaceutical Quality, closed the forum presenting on ICH Q12 implementation and how it can improve drug product quality, enhance company operations and reduce the need for regulatory oversight. She discussed the tools available to industry in ICH Q12, emphasizing the importance of determining established conditions using effective pharmaceutical quality system (PQS) principles, performing effective criticality assessments and developing appropriate control strategies. She said generic firms have the knowledge to develop established conditions and that established conditions can be submitted to an application after approval; they need not be added to every section of an application.
Finally, Boam addressed the confusion surrounding established conditions. She touted the work of the Established Conditions Coordinating Committee and the ICH Q12 Assessment Implementation Team to ensure consistent assessment of established conditions within the FDA.
The Generic Drugs Forum was well organized and addressed key topics affecting the generics industry. Presentations were to the point and provided excellent examples of the work FDA is accomplishing in the generic drug arena, providing information on:
- FDA accomplishments in generic drugs for 2021 • KASA program, a detailed explanation and how the information will be used to evaluate ANDAs
- Role of data integrity in review of ANDAs and how firms can prevent data integrity issues by having a culture of quality
- Issues with generic drug quality and drug development
- Best practices to implement ICH Q12 and tools used to improve ANDA drug product quality and facility assessments.
OPQ Director, Michael Kopcha, PhD, summarized CDER’s strategic vision: To ensure the present and future of pharmaceutical quality, the FDA is focusing on effective facility and application assessments, improving the industry’s quality management maturity and developing advanced manufacturing within the industry.
Slides and recordings of the 2022 Generic Drugs Forum are available.



 David Jaworski is PDA’s Sr. Advisor for Scientific Affairs. He was a Senior Policy Advisor at U.S. Food and Drug Administration for more than seven years and had previously worked in the CDER Office of Compliance as a Consumer Safety Officer and as Acting Branch Chief in the Domestic Case Branch. His experience in the pharmaceutical industry encompasses 22 years in business management, research, manufacturing and consulting.
David Jaworski is PDA’s Sr. Advisor for Scientific Affairs. He was a Senior Policy Advisor at U.S. Food and Drug Administration for more than seven years and had previously worked in the CDER Office of Compliance as a Consumer Safety Officer and as Acting Branch Chief in the Domestic Case Branch. His experience in the pharmaceutical industry encompasses 22 years in business management, research, manufacturing and consulting.