How to Establish Effective CCS with TR-90
The manufacturing of medicinal products is critical to minimize the likelihood of contamination ingress into the product flow path. This is especially true in the production of sterile medicinal products that cannot undergo terminal sterilization. The August 2022 revision to European Union Annex 1: Manufacture of Sterile Medicinal Products was a significant change in the expectations of industry members and has impacted the biopharmaceutical industry as it pertains to the specific and integral parts of pharmaceutical product manufacturing that is centered around a proper contamination control strategy (CCS). The Annex 1 glossary defines a holistic CCS as “A planned set of controls for microorganisms, endotoxin/pyrogen, and particles, derived from current product and process understanding that assures process performance and product quality. The controls can include parameters and attributes related to active substance, excipient, and drug product materials and components, facility and equipment operating conditions, in-process controls, finished product specifications, and replacing equipment components.” This explanation of CCS gives manufacturers a complete approach regarding CCS, which is one of the more significant additions to the revision of Annex 1 compared to the 2008 version (see Table 1).
Nonetheless, CCS is not a new concept, as manufacturers have employed contamination controls for decades to mitigate the impact on the product, minimizing product loss and producing high-quality medicinal products for patients. Now, regulatory authorities have placed the CCS at the forefront of sterile product manufacturing.
The Publication of TR-90
The recently published PDA Technical Report No. 90: Contamination Control Strategy Development (TR-90) in February 2023 presents the CCS through a combination of these linked elements: foundation, contamination controls, validation of controls, monitoring of controls and governance, demonstrating the CCS in a methodical manner (see Figure 1). Industry subject matter experts wrote this technical report to provide guidance on how to establish an effective CCS. The report is applicable for new, existing or retrofitted facilities or processes. The CCS focuses on practices associated with the control of microorganisms, endotoxins/pyrogens and particulate matter in the manufacturing of sterile drugs. The CCS, however, can be used for processes for low bioburden drug substances and nonsterile drugs as well.
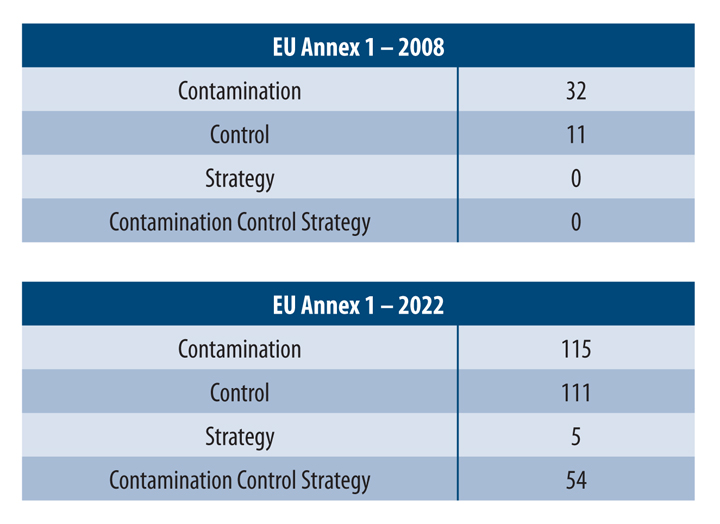 Table 1 Word Comparison between 2008 Annex 1 and 2022 Annex 1
Table 1 Word Comparison between 2008 Annex 1 and 2022 Annex 1
 Figure 1 Elements of a Contamination Control Strategy
Figure 1 Elements of a Contamination Control StrategyFoundations
TR-90 establishes foundations for the CCS that are crucial to developing, implementing and continuously improving an effective CCS. This includes scientific knowledge, QRM and personal awareness/quality culture.
Scientific knowledge encompasses two different but equally important aspects – process knowledge and technical knowledge. These two types of knowledge form the initial foundation of the CCS. Process knowledge is critical to ensure a robust understanding of the specific manufacturing steps and the potential for contamination ingress, proliferation, reduction and removal associated with the manufacturing process. Furthermore, technical knowledge is critical to understanding the mechanisms that may be employed to prevent, reduce and remove contamination.
Quality risk management (QRM) is a systematic process for assessing, controlling, communicating and reviewing risk that is to be employed in the manufacture of medicinal products. In addition, QRM is to be used in new and existing processes and facilities to identify and assess the risk of contamination ingress. Contamination control risk analysis should identify which individual controls are unacceptable, and the CCS must be designed with redundant individual control elements to ensure a single failure will not result in a contamination event.
Personnel awareness/quality culture must be a priority for companies to ensure a proper understanding of the CCS. The firm should have a dedicated champion or a cross-functional team to oversee the overall performance of the CCS (the impact of a cross-functional team will be discussed more in the governance section). In addition, personnel awareness of the CCS can, directly and indirectly, impact the strategy each employee employs, and a company’s strong quality culture will ensure that contamination control is a priority.
The foundational elements discussed in TR-90 align with what industry enablers described in International Council for Harmonization Quality Guideline Q10: Pharmaceutical Quality Systems. Therefore, the CCS must be established based on the identified foundations discussed in PDA TR-90 to ensure the reliability of the CCS.
Contamination Controls
Contamination controls are the pillars of the CCS and must be identified early in the development of new manufacturing processes. The use of the foundational elements in the development and implementation of the contamination controls will ensure a high-functioning CCS. Furthermore, the contamination controls are designed to utilize the fundamental elements described in the foundations section to prevent, then mitigate contamination ingress. These controls include container closures, consumables, design (e.g., facility, process, utility), equipment, materials, personnel and vendors to ensure all interconnected linkages are recognized to establish a strong CCS.
Incorporation of proper risk-based designs for equipment, facility, utility and process are essential to proper CCS integration. Equipment of poor design adds risk and may lead to contamination ingress and proliferation. During routine and ongoing use, microbial control of equipment is vital to maintain its functional condition. Moreover, throughout a production area's initial design (or re-design) phase, the facility and utility systems must incorporate key elements of the CCS. Proper facility designs provide the appropriate production environment through multiple design aspects (e.g., pressure cascades, segregation, flow). The manufacturing processes should be designed to prevent contamination ingress from microorganisms, endotoxins/pyrogens and foreign particulates.
Material and waste pathways must be defined within the CCS. Facility cleaning and disinfection are imperative for the holistic CCS to minimize human-borne and transient contamination from equipment transfer. The transfer of materials and equipment within and between the manufacturing zones must be properly defined and repeatable to ensure the mitigation of contamination ingress.
Personnel is the number one source of microbiological contamination within the manufacturing environment. This requires that multiple contamination controls be defined within the CCS according to the individual steps in the manufacturing process. Personnel controls (e.g., people flow, gowning, hygiene) must be identified within the CCS to minimize contamination ingress.
Materials (e.g., raw materials, active pharmaceutical ingredients, excipients, containers, filters) may be vectors for contamination ingress in the manufacturing process. Due to this risk, vendor management is a critical component of the CCS. All materials must be identified with appropriate specifications to minimize the impact of contamination within the product flow path. This includes properly evaluating of a supplier's quality systems, such as storage, packaging, testing and transporting materials.
Validation of Controls
The pillars of contamination control must be properly qualified and validated to ensure proper contamination control is maintained throughout routine processing and use within the defined manufacturing space. This qualification and validation should be risk-based and appropriate for analytical methods, equipment, facility, processes and personnel. Also, materials that undergo quality control testing must have validation associated with all methods. If a third party executes the testing of materials, the method validation should be included in the quality agreement with the provider, and the lifecycle of the testing must be included within the scheduled CCS review.
Good qualification and validation for manufacturing equipment, facilities and processes should be appropriately defined within the CCS and be risk-based. There should be a defined risk-based approach to routine re-qualification, including documentation of the equipment's lifecycle, facilities and processes. The qualification interaction between personnel and the equipment and facility is an important piece of the CCS and should be identified for all necessary processes. This should also include an approach to personnel re-qualification for risk-based activities within the processes.
Monitoring of Controls
Monitoring controls are a central part of a holistic CCS, as they provide feedback on the contamination controls, including, but not limited to, the design, validation and qualification of equipment, facilities and process. Consequently, Monitoring controls should be based on sound scientific principles, risk assessment and regulatory requirements.
Several monitoring controls (e.g., personnel, in-process, material, environmental, utility) may be captured through multiple mechanisms (e.g., gauges, growth media, probes, sensors). This data should be continuously evaluated to determine the appropriateness
and the performance of the associated contamination control(s).
Monitoring controls, while a key part of the holistic CCS, need to be understood based upon what contamination control the data is representing. Not all data is directly linked to a failure of a single (or multiple) control, but a rather
normal variation within a robust program.
Governance
The governance of the CCS shall include inputs from multiple aspects of the quality management system (e.g., CAPA, change management). The CCS should be considered a “living” document that is periodically reviewed to ensure all components of the strategy are functioning as expected. The CCS requires a defined governance structure to oversee the program's overall effectiveness.
This governance structure must include a cross-functional team with the appropriate authority to oversee the program and mechanisms that allow escalating adverse trends and events. Oversight performed by the cross-functional team should include ongoing performance reviews of the CCS at regular intervals (e.g., weekly, monthly, quarterly) based on identified key process indicators. Adverse trends and events associated with the CCS, which may occur occasionally, should be quickly communicated to the cross-functional team, who will respond appropriately. If firms have a robust knowledge management program for all manufacturing processes, this will ensure historical events and trends are maintained and incorporated into future process modifications.
Conclusion
Lastly, because of TR-90 and its elements above, creating a sturdy CCS will allow an organization to assess and continuously improve its level of contamination control, which can reduce manufacturing losses and better attend to the patients the industry serves.



 Fred Ayers, is
a contamination control/sterility assurance expert with over 20 years of experience in various roles with quality assurance, quality control, and technical services. He has been responsible for ensuring Quality Standards and associated contamination
controls/sterility assurance programs evolve with global regulatory expectations. Fred is a technical leader with an external focus and engagement throughout the biopharmaceutical industry to influence the direction of regulatory expectations.
He was a Task Force member and co-author of PDA TR-90. Fred has been a strong supporter and contributor to PDA. He has been a PDA Midwest Chapter Board Member since 2014, serving as Chapter President from 2020-2021, and is a current member
of the PDA Scientific Advisory Board.
Fred Ayers, is
a contamination control/sterility assurance expert with over 20 years of experience in various roles with quality assurance, quality control, and technical services. He has been responsible for ensuring Quality Standards and associated contamination
controls/sterility assurance programs evolve with global regulatory expectations. Fred is a technical leader with an external focus and engagement throughout the biopharmaceutical industry to influence the direction of regulatory expectations.
He was a Task Force member and co-author of PDA TR-90. Fred has been a strong supporter and contributor to PDA. He has been a PDA Midwest Chapter Board Member since 2014, serving as Chapter President from 2020-2021, and is a current member
of the PDA Scientific Advisory Board.