How to Get Your ATMP From the Lab to the Market
What is actually involved in taking an advanced therapy medicinal product (ATMP) from a brilliant idea in the lab to a successful product on the market? Is it similar to the development of a combination product? Or a monoclonal antibody? Just how difficult can it be? The level of activity surrounding ATMPs has been increasing, with some remarkable therapeutic opportunities currently being explored.
First, let me begin with a definition: “advanced therapy medicinal products” (or ATMPs) is a regulatory term used in Europe for medical products based on genes (gene therapies), cells (cell therapies), and tissues (tissue engineering). “Regenerative medicine” is another often-heard term that describes a similar range of products. ATMPs are complex and diverse, with varying requirements, both for manufacturing and delivery. Cells are living entities, and a comparison with biologics can be misleading; human DNA has a molecular weight of 2.2 TDa, i.e., 15×106 × the molecular weight of adalimumab (Humira®), which alters our view of delivery options.
Getting to a Realized Product
For an ATMP product to be successful, the No. 1 usual requirement is that it be clinically effective, meaning the clinical outcome results in a measurable improvement in the patient’s condition. Equally important future matters need to be considered, however. How will the product or therapy actually be applied or delivered? Who will deliver or administer the therapy? What form could any delivery device or system take? There are more questions when it comes to manufacturing. How could it be manufactured? What are the GMP implications and considerations? What should the objectives be regarding capability/robustness of the design? And the bottom line: what will the cost of goods be—and do we understand what the market acceptable price is likely to be?
For an ATMP product to be successful, the No. 1 usual requirement is that it be clinically effective, meaning the clinical outcome results in a measurable improvement in the patient’s condition. Equally important future matters need to be considered, however. How will the product or therapy actually be applied or delivered? Who will deliver or administer the therapy? What form could any delivery device or system take? There are more questions when it comes to manufacturing. How could it be manufactured? What are the GMP implications and considerations? What should the objectives be regarding capability/robustness of the design? And the bottom line: what will the cost of goods be—and do we understand what the market acceptable price is likely to be?
Rational, objective answers to these questions must be based on more than a rose-tinted dream of what a “great idea” or “clever science” can achieve. While I do not want to pour cold water on the promise offered by any brilliant/innovative science, serious consideration of the practical means of manufacturing and applying the product or device is equally important.
First, let me acknowledge that innovation is a creative process, of which individual genius, the creative spark, and eureka moments are just initial steps. But the questions of “How do we make it?” and “How will it be delivered?” are as essential to this process as the science. See Figure 1 for an example of what happened when one company prioritized the initial innovation over the process.
Teamwork, Teamwork, Teamwork
The nature of ATMPs and regenerative medicines demands bringing deep biological knowledge and complex engineering skills to the development team (1). This blending of skillsets is difficult to achieve but necessary for any organization to succeed. To bring ideas from the lab bench to commercial reality requires engineers and designers who understand biology and clinical science as well as biologists and clinical scientists who understand engineering and design (2). Team members must be willing to share, engage, and respect each other across the spread of disciplines and skills. An Innovation Management approach (already proven across a wide range of sectors), embraces a mindset and guiding philosophy that suits ATMP development extremely well. As applied within my own organization, Innovation Management describes a healthy mix of attributes and inputs from the overall development team: creativity (freedom to explore, develop, and suggest new ideas), science and supportive/explanatory theory (ability to understand and validate concepts and potential solutions), and structural formality (procedures for capturing actual achievement and focusing on shared goals and objectives).
It is important to recognize that all these attributes and inputs—creativity, science, and structure—need to be present throughout the development process, but the ratios will vary as development progresses. At the front end, plenty of creativity, backed by enough science (and a light touch of structure) and theory, helps pick out promising approaches and close down dead ends. Later stages demand a lot of structure, informed by sound theory for validation, with more limited opportunity for creativity.
Three Critical Attributes Necessary
Clinical efficacy starts the process. The next steps for a successful product are usability and manufacturability. For ATMPs, unless all three attributes are taken seriously, a therapy may be, at best, suboptimal—or, at worst, a disaster.
Usability, its assessment and improvement, is the primary objective of human factors engineering. Put simply, it’s about making sure that a product, device, or system is easy to use correctly and difficult to use incorrectly. Standards and guidance documents for analytical and empirical human factors engineering include ISO 62366, ANSI/AAMI HE75, and the US FDA’s guidance, Applying Human Factors and Usability Engineering to Medical Devices. Human factors engineering should inspire and inform innovation and design.
Manufacturing is often the forgotten guest, invited in when the celebrations have come to a halt because no one remembered to bring a corkscrew! ATMPs often require developing new tools, techniques, and processes, and the initial vision of the new therapy has to acknowledge that process engineering, GMPs, device engineering, and regulations are as crucial as good science. Clarity on several key matters must be achieved early on, such as who will be responsible for design and manufacturing and how will the product be packaged, sterilized, and shipped? I recommend also looking at processes and techniques from different industries (e.g., food, electronics, automotive, etc.) from a GMP perspective.
A couple of final words regarding both usability and manufacturing. There is no shame in requesting expert help; a great deal can be gained from early engagement with “outsiders” in human factors engineering, manufacture, and design. Usability and manufacturability are not something that can be ignored until the end of development.
Most ATMPs begin existence in the lab, using manual methods and protocols. It can be expected that human variability may conceal either latent weakness or potential promise, especially as the development progresses. Early, critical, and objective assessment of the status of the product and what has been achieved is essential. Technology Readiness Levels were originally used in the defense industries to judge the development status of weapons systems and aircraft. They are equally valid in assessing the true status of medical development projects (Table 1). The “valley of death” defines the transition from proof of concept to proven capability in manufacture and ensures stakeholders share a common appreciation of what challenges have been met and where resources need to be applied.
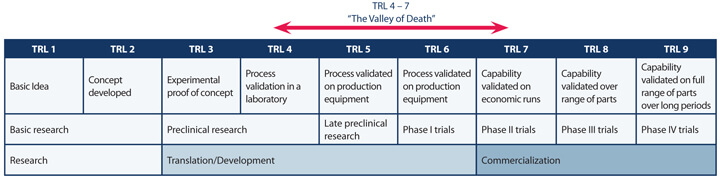
It can also prove very useful to apply some early, simplified automation steps to help screen out erroneous effects. It’s human nature to nurse our pet projects through the lab, such that real performance potential or issues are never observed. Simple, “manumatic” streamlining of processes can help set the template for later development stages.
The Development Process for ATMPs
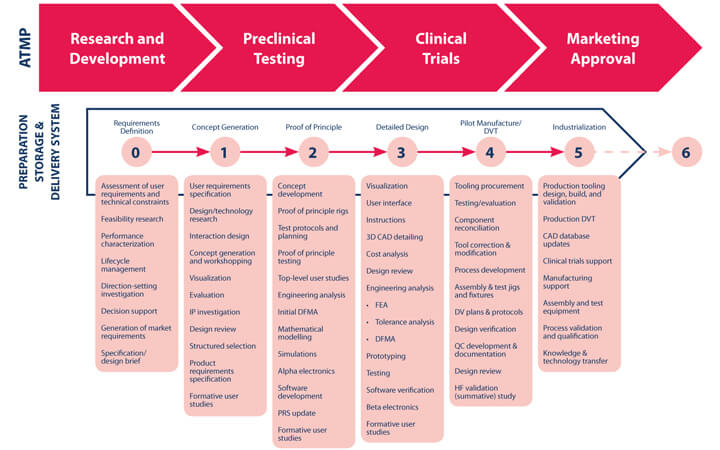
The ideal point to begin considering manufacture, storage, and delivery is during, or even before, preclinical testing. It becomes very difficult, time-consuming, and expensive to make changes once clinical testing is underway. Figure 2 shows a typical device development process overlaid against the ATMP development process (transition from TRL 3-4 falls within Stage 2, Proof of Principle; transition from TRL 6-7 falls within Pilot Manufacture/DVT, Stage 4).
It makes no sense to delay consideration of the manufacturing process and delivery method, since these aspects often define the ATMP. Without early consideration of design, engineering, and manufacture of the product and means of delivery, an ATMP may never emerge as a product, commercially or therapeutically, as shown in Figure 3.
Though ATMP development processes differ from those for production and delivery systems, success depends substantially on the latter.
The wide variety of therapy types, delivery/application requirements, and manufacturing processes often means there is a very limited scope for making use of off-the-shelf production and delivery solutions. Furthermore, clinical trials usually look very different to those for pharma products, especially as ATMPs may be produced in very small batches (possibly down to n=1).

Conclusion
Innovative science, product engineering, and commercial imperatives must all be integrated to achieve a successful ATMP. The harsh realities of bringing a breakthrough ATMP to the market won’t go away if we ignore them—we will just experience failure and lost opportunity. Assessing the clinical benefit of a potential ATMP must be accompanied by more intelligent product development focused on improved manufacture.
The wisdom of involving all stakeholders early on should be obvious. If we ignore the role each stakeholder plays, the outcome will suffer. Improving our ability to judge objectively the clinical, technical, and commercial potential of a great idea has to be beneficial to the development process and to those involved. A range of applicable tools and techniques are available. But these tools sit alongside more fundamental characteristics of proven value in development and working as a team—honesty, thoroughness, technical rigor...and now and again, a touch of humility, since none of us know all the answers!
[Editor’s Note: The author presented this at last year’s PDA ATMP conference in Berlin.]
Acknowledgements to Richard Archer of TwoBC Ltd, and to Ben Wicks and Vicky Shipton at Team Consulting
References
- Livesey, F. “Enabling the emergence of the regenerative medicine industry in the UK.” Centre for Economics and Policy, Institute for Manufacturing, University of Cambridge Engineering Department. March 2008. www.ifm.eng.cam.ac.uk/uploads/Research/CIG/Workingpaper3.pdf (accessed Feb. 24, 2017).
- “Regenerative medicine - Science and Technology Committee Contents,” UK Parliamentary Business, 2013. www.publications.parliament.uk/pa/ld201314/ldselect/ldsctech/23/2306.htm (accessed Feb. 24, 2017).



