Keep Aseptic Conditions Continuity within Transfer Procedures A Smart CCS

The transfer of sterile materials within the aseptic processes remains a thorny point to address and requires robust risk assessment and risk management within a comprehensive contamination control strategy (CCS).
As well emphasized in EU GMP Annex 1: Manufacture of Sterile Medicinal Products and in §4.10 of the World Health Organization Annex 2: WHO Good Manufacturing Practices for Sterile Pharmaceutical Products, “The transfer of equipment and materials into and out of the cleanrooms and critical zones is one of the greatest potential sources of contamination” (1,2).
All the essential manual practices involved require a punctual consideration of all potential risks related to contamination protection. That includes procedures, training, validation and controls, as well as the potential contribution to the particulate contamination of the protective materials.
Class/ISO 5 maintenance and continuity is an important point of aseptic production. As these guidelines clearly state, "...Where possible, items should be sterilized and passed into these areas through double-ended sterilizers [§4.11]. The use of rapid transfer port technology should also be considered” [§8.47] (1,2).
Direct Transfer
Implementation of best practices in contamination control is the best approach for materials transfer. A combined set of technological solutions aimed to avoid all critical steps by using a smart design to achieve more efficient control of the contamination within the CCS is paramount.
An interesting example is direct transfer by a magnetic-driven door (e.g., Magnetodoor®) designed and created to provide a direct connection with an isolator, as shown in Figure 1. It is a special sliding door which, compared to conventional doors that require wheels, toothed belts and other mechanisms, does not release any particles due to its different design.
This magnetic-driven door does not have any hidden points, which represent a typical criticality for cleaning, and allows a minimization of the space and spare mechanical parts needed compared to a conventional door with a swing-and-book opening movement. In such a way, the prevention of potential contamination during the transfer is ensured by design, creating a direct and integral connection between a sterilizer and/or combined washer and sterilizer units and the isolator.
This configuration not only manages risks but, where possible, eliminates them. This looks like a good approach, considering the introduction of the European Medicines Agency Guideline on the Sterilization of the Medicinal Product, Active Substance, Excipient and Primary Container: “Sterility cannot be assured by testing; it needs to be assured by the use of a suitably designed, validated and controlled manufacturing process” (3).

Several configurations can be designed and customized with various volumes, adaptable to different process needs (from 150 liters up to 85 cubic meters), but, in general, there are three major types of direct connections:
- Connection to the load side — The connection on the load side of the isolator (see Figure 2) includes a double magnetically operated door. The unit can be a pass-box for decontamination or a sterilizer, depending on the flow of the production process.
- Connection to the exhaust side — The connection on the discharge side of the isolator (see Figure 3) always includes the double magnetically operated door and allows the articles to be directly unloaded from
the isolator into a sterilizer.
The sterilizer unit can foresee different sterilization methods, and this solution allows the terminal sterilization of sealed packages.
- Side (lateral) connection as service peninsula — This lateral connection (see Figure 4) includes one or two magnetically operated doors and was conceived considering the eventual need to move the articles from
the isolator to the sterilizer and back into the isolator.
This configuration facilitates the direct movement with class continuity of auxiliary items used in processing (e.g., in a filling line installed inside the isolator) directly from the isolator into a sterilizer and the return of the items to the same isolator once they are sterilized. Beyond the elimination of all risks related to transfers, this solution guarantees a clear advantage of space reduction.
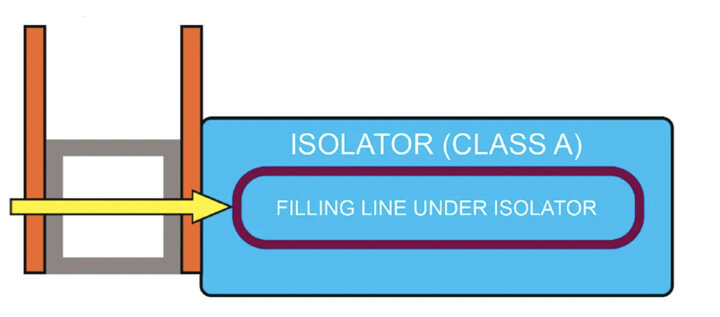
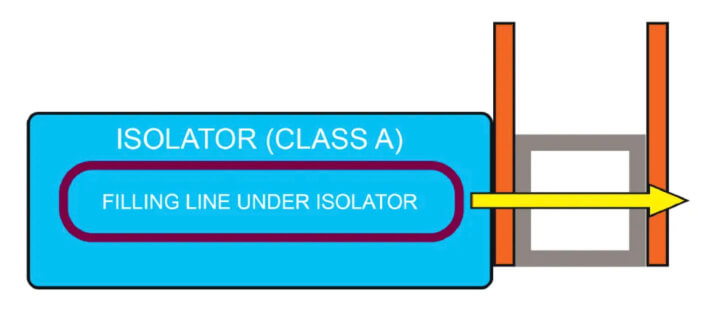
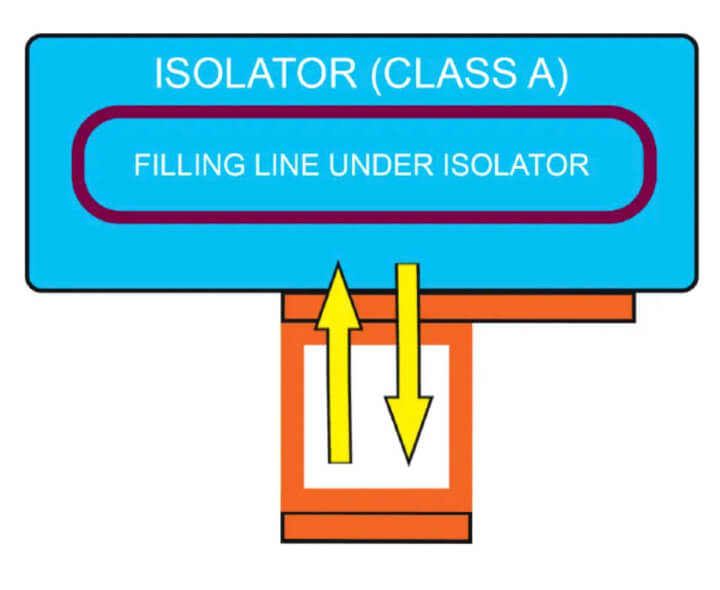
All choices allow to move ancillary items directly to and from the isolator chamber without the need to take them out from the isolator in the cleanroom, and it is always possible, if necessary, to combine the unit with the washing machine.
By eliminating the transfer risks, and optimizing the space in the cleanroom and the process operations, the use of the direct connection to the isolator through a magnetic-driven door represents an effective solution for smart GMP compliance.
Low-Temperature Sterilization Technology
I believe few are aware of the new low-temperature hydrogen peroxide sterilization technology. I am talking about sterilization and not about the common superficial decontamination by contact!
HyPerPure® technology is a cold sterilization process able to reach up to a LOG12 reduction within all points of the load, under deep vacuum and zero air. How can it be so efficient and still be able to achieve such a strong “killing” effect?
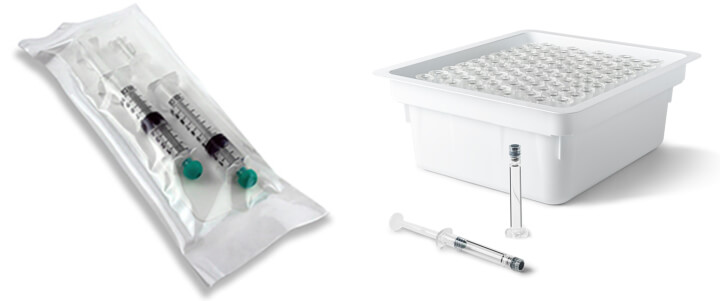
The peculiarity resides in a deep vacuum combined with a particular H2O2 ionization (i.e., patented peroxide generator). This permits a strong penetration of hydrogen peroxide, even through Tyvek envelopes or, if needed, inside any object, including those with complex geometries and small lumens (see Figure 5).
This is a different situation compared to the decontamination process.
Except for materials proven noncompatible with vaporized H202, this low-temperature sterilization process could provide an interesting solution for all thermosensitive or thermolabile products (e.g., free or wrapped, medical devices, plastic syringes). It could also be an interesting alternative to ethylene oxide (EtO) or gamma-ray sterilization, while taking advantage of an easy internalization within the pharmaceutical facility.
In addition, being a low-temperature technology, this form of sterilization can speed up the process (reduced to three to eight hours, depending on the loads), optimizing the process and reducing the costs.
Conclusion
The U.S. Food and Drug Administration continues to encourage finding alternatives to ethylene oxide sterilization. In January 2024, the Agency included hydrogen peroxide in the list of Class A sterilization technologies for medical devices and saturated steam, dry heat and radiation (4,5).
With the recognition of ISO 22441:2022 Sterilization of Health Care Products — Low Temperature Vaporized Hydrogen Peroxide — Requirements for the Development, Validation and Routine Control of a Sterilization Process for Medical Devices (6), a low-temperature vaporized hydrogen peroxide process like HyPerPure® became an interesting, available alternative sterilizing technology—green, environmentally friendly and widely applicable.
In terms of sustainability, if low-temperature vaporized hydrogen peroxide is compared to steam-vapor sterilization, the cost reduction is surprising: 70% energy saving, 70% reduction of carbon dioxide emissions and practically a 100% water savings!
The possible combination of an innovative design and a new low temperature demonstrate how much this technology could help move one more step toward compliance with “continuous improvement.”
References
- Annex 1: Manufacture of Sterile Medicinal Products, European Union Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use (22_08_2022); https://health.ec.europa.eu/system/files/2022-08/20220825_gmp-an1_en_0.pdf Annex 2: WHO Good Manufacturing Practices for Sterile Pharmaceutical Products, Technical Report Series 1044, 2022; https://www.who.int/publications/m/item/trs1044-annex2
- European Medicines Agency Guideline on the Sterilisation of the Medicinal Product, Active Substance, Excipient and Primary Container; https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-sterilisation-medicinal-product-active-substance-excipient-and-primary-container_en.pdf
- FDA Facilitates Broader Adoption of Vaporized Hydrogen Peroxide for Medical Device Sterilization, Agency Continues to Encourage Ethylene Oxide Sterilization Alternatives https://www.fda.gov/news-events/press-announcements/fda-facilitates-broader-adoption-vaporized-hydrogen-peroxide-medical-device-sterilization
- FDA Adds Vaporized Hydrogen Peroxide as Sterilization Alternative to EtO; https://www.medtechdive.com/news/fda-guidance-vaporized-hydrogen-peroxide-sterilization/704316/
- ISO 22441:2022Sterilization of health care products — Low temperature vaporized hydrogen peroxide, https://www.iso.org/standard/73214.html
- J. Markarian, Aseptic Processing Innovations on Display, April 17, 2019; http://www.pharmtech.com/aseptic-processing-innovations-display



