Modern Microbial Methods Supporting a Contamination Control Strategy
[Author’s note: The authors are part of a collaboration of industry working groups that joined forces in 2021 to support the awareness and adoption of modern microbial methods. These groups include the BioPhorum Operations Group, the Kilmer Community Rapid Microbiology Methods group, the Online Water Bioburden Analyzer working group and the Process and Environmental Monitoring Methods (PEMM) working group.]
The EU GMP Annex 1: Manufacture of Sterile Medicinal Products addresses the manufacture of sterile medicinal products and includes the requirement for a documented contamination control strategy (CCS) (1).
At a high level, the CCS should outline the scientific evidence leveraged to support the prevention and detection control measures that enable successful aseptic manufacturing. While concepts related to contamination control are not new to the industry, the emphasis on using modern microbial methods is a new addition to the guidance. Annex 1 now underscores the importance of using validated and reliable methods for monitoring and controlling microbiological contamination in sterile product manufacturing. This article outlines the types of modern methods currently available and where they can be implemented to align with the principles outlined in Annex 1 to support the quality and safety of sterile medicinal products.
Background
The term modern microbial method (MMM) is used to describe a method that is an alternative to or an enhancement of the compendial agar-based method. Other similar terms used to describe such methods are rapid microbiological methods and alternative methods, as used in Annex 1 (1). These methods can offer advantages over the compendial method, including but not limited to a shorter time to detection, real-time reporting of results, continuous monitoring, higher sensitivity and a lower false negative rate (e.g., due to detection of viable but not culturable (VBNC)) (2). Such advantages can be used to better support the detection of contamination and its prevention through a better understanding of the environment than intermittent sampling with the compendial method might provide.
MMM includes technologies based on the use of intrinsic fluorescence, extrinsic fluorescence (e.g., viability staining), bioluminescence, enzyme indicators, Raman spectroscopy, flow cytometry, solid phase cytometry, polymerase chain reaction (PCR) and automated colony detection and counting. Although described as modern compared to a method that has been used for over a century, many of these alternative methods are based on technologies that have been used for decades.
The CCS elements discussed in Annex 1 include the design of both the facility and manufacturing process, premises and equipment, personnel, utilities, and raw material controls - including in-process controls, product containers and closures, vendor approval, management of outsourced activities, process validation, validation of sterilization processes, preventive maintenance, cleaning and disinfection, monitoring systems (including alternative methods), prevention mechanisms and continuous improvement (1). As it is intended in this article to communicate the elements of a CCS that MMMs can support, the CCS elements from Annex 1 have been combined into the following four categories with investigational tools having application to all:
- Facility (includes premises and equipment, utilities and environmental monitoring)
- Personnel and training
- Raw materials (includes raw material controls and product containers and closures)
- Process (includes process controls, process validation, validation of in-process sterilization, preventive maintenance, cleaning and disinfection)
- Investigational tools (prevention mechanisms in Annex 1)
Modern Microbial Method Technologies
MMMs help to revolutionize the landscape of pharmaceutical quality control by countering known deficiencies of traditional culture-based techniques. In the pharmaceutical industry, where stringent regulations and product safety are paramount, adopting MMM can help companies react faster to contamination events and release finished products sooner. For example, receiving rapid sterility result within days or even hours significantly reduces turnaround times compared to traditional methods. In addition, MMMs often minimize the need for extensive sample preparation. They can reduce the risk of false negatives associated with visual determination of microbial growth, improving the reliability and integrity of microbial testing results. This enables pharmaceutical companies to make timely decisions and take corrective actions to ensure product quality and compliance with regulatory standards.
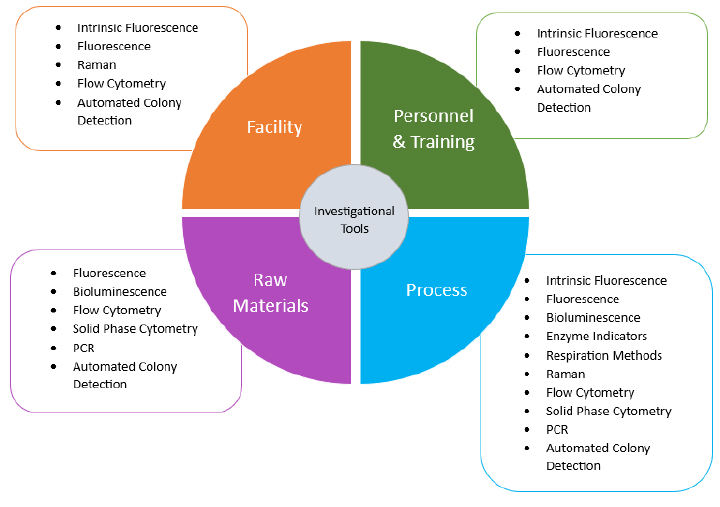
As the pharmaceutical industry continues to embrace technological advancements, the widespread adoption of MMM underscores its pivotal role in ensuring the safety, efficacy and compliance of pharmaceutical products (see Figure 1). Examples of MMM technologies and the elements of a CCS that they support can be found in Table 1.
Table 1 General MMM Technologies and Their Applicability to Elements of a CCS| General Technology | Mode of Action (3) | CCS Element* Applicable To |
|---|---|---|
| Intrinsic fluorescence and Mie scatter | Measurement of total and biologic particles in air or water through detection of intrinsic fluorescence | Personnel and training, facility, process, investigations |
| Fluorescence (e.g., Viability staining) | Measurement of total particulate and viable cells in air or water through detection of extrinsic fluorescence | Personnel and training, facility, process, raw materials, investigations |
| Bioluminescence | Measurement of viable organisms in sterile and non-sterile samples | Process, raw materials, investigations |
| Enzyme Indicators | Measurement of bio-decontamination process using gaseous hydrogen peroxide | Process, investigations |
| Respiration Methods | Measurement of sample changes resulting from microbial respiration (e.g., CO2-related changes in color/fluorescence, pressure changes) | Process |
| Raman | Spectral signature of each particle is obtained for the identification and enumeration of organisms through comparison to a library of known microorganism signatures | Facility, process, investigations |
| Flow Cytometry | Measurement of intrinsic or extrinsic fluorescence to enumerate viable counts | Personnel and training, facility, process, raw materials, investigations |
| Solid Phase Cytometry | Viability or species-specific stains are used with resulting fluorescence detection to enumerate bioburden | Process, raw materials, investigations |
| Polymerase Chain Reaction | Detection of specific species for testing water, wastewater, in-process samples and raw materials | Process, raw materials |
| Automated Colony Detection | Colony- forming unit enumeration through detection of auto-fluorescence and growth using optics/camera | Personnel and training, facility, process, raw materials |
Evaluation and Implementation of a Modern Microbial Method
There is significant encouragement and support within the industry for the use of MMM. In addition to Annex 1, industry guidance documents like the PAT — A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance (4), USP General Chapter <1223> Validation of Alternative Microbiological Methods (5), Eur. Ph. Chapter 5.1.6 (6) and PDA’s Technical Report No. 33: Evaluation, Validation and Implementation of Alternative and Rapid Microbiological Methods (7) have encouraged using advanced technologies and supported their validation. Furthermore, industry collaborations and regulatory emerging technology programs, such as the U.S. Food and Drug Administration Emerging Technology Program and European Medicines Agency Innovation Task Force, are also available to support new technologies.
In addition, understanding the goals and needs of one’s organization can aid in determining which modern method(s) to investigate further. Knowledge of a technology’s capabilities and breadth of application are important considerations. As shown in the table above, some modern technologies may support multiple elements of a CCS. An evaluation of available technologies and implementation path may include:
- Initial technology assessment – company goal and need alignment, applications, and ease of implementation
- Technical considerations – technology capabilities, limitations and data review
- Data and compliance risk – connectivity, data retrieval and 21 CFR Part 11
- Cost considerations – initial and long-term
- Overall instrument evaluation
Conclusion
Numerous MMMs are available to support CCS within the pharmaceutical market. These technologies can offer broader applicability to CCS elements and improved detection and monitoring capabilities than the traditional method alone. An introduction to the general technologies is provided above, along with the elements of a CCS that these technologies may support. Assessing how such technologies may fit into one’s company goals and needs is highly encouraged, particularly related to a CCS.
References
- EU GMP Annex 1: Manufacture of Sterile Medicinal Products https://www.gmp-compliance.org/files/guidemgr/20220825_gmp-an1_en_0.pdf
- Newby, Paul. The Significance and Detection of VBNC Microorganisms. American Pharmaceutical Review, 2007. https://www.americanpharmaceuticalreview.com/Featured-Articles/113051-The-Significance-and-Detection-of-VBNC-Microorganisms/
- Miller, M. The RMM Product Matrix. http://rapidmicromethods.com/files/matrix.php
- U.S. Food and Drug Administration. Guidance for Industry PAT – A Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance, Sept. 2004
- U.S. Pharmacopeial Convention. General Chapter <1223> Validation of Alternative Microbiological Methods. In USP 42–NF 37; USP: Rockville, Md. 2016.
- Council of Europe. Alternative Methods for Control of Microbiological Quality Chapter, 5.1.6. In European Pharmacopoeia 10.0, Council of Europe: Strasbourg, 2019; p 628.
- Parenteral Drug Association. Technical Report No. 33 (Revised 2013): Evaluation, Validation and Implementation of Alternative and Rapid Microbiological Methods; PDA: Bethesda, Md., 2013.



 Allison Scott is a Staff Scientist at Particle Measuring Systems, a member of the PEMM working group and a facilitator and member of the M3 Collaboration.
Allison Scott is a Staff Scientist at Particle Measuring Systems, a member of the PEMM working group and a facilitator and member of the M3 Collaboration.
 Timothy Cser is a Senior Technology Specialist with Millipore Sigma and a member of the M3 Collaboration.
Timothy Cser is a Senior Technology Specialist with Millipore Sigma and a member of the M3 Collaboration.
 Jon Kallay is a Senior Scientific Portfolio Specialist at Charles River Laboratories and a member of the M3 Collaboration.
Jon Kallay is a Senior Scientific Portfolio Specialist at Charles River Laboratories and a member of the M3 Collaboration.
 Amanda McFarland is a Senior Consultant at ValSource, Inc., a member of the Kilmer Community Rapid Microbiology Methods group, Co-lead of the Kilmer Regulatory Innovations group and a member of the M3 Collaboration.
Amanda McFarland is a Senior Consultant at ValSource, Inc., a member of the Kilmer Community Rapid Microbiology Methods group, Co-lead of the Kilmer Regulatory Innovations group and a member of the M3 Collaboration.
 Nina Moreno is the Director of Lab Operations at Nelson Laboratories, LLC, a member of the Kilmer Community Rapid Microbiology Methods group and a member of the M3 Collaboration.
Nina Moreno is the Director of Lab Operations at Nelson Laboratories, LLC, a member of the Kilmer Community Rapid Microbiology Methods group and a member of the M3 Collaboration.
 Meghan Provenzano is the Global Product Manager for Biodetection at Veolia Water Technologies and a member of the M3 Collaboration.
Meghan Provenzano is the Global Product Manager for Biodetection at Veolia Water Technologies and a member of the M3 Collaboration.