PDA Capital Area Chapter Roundtable on Complete Response Letters Draws a Large Sunday PM Audience
Nearly 80 industry experts participated in the PDA Capital Area Chapter Roundtable – FDA Complete Response Letters: A Growing Industry Challenge with Big Impacts on a sunny and pleasant Sunday afternoon. Eschewing leisurely strolls on the U.S. Capitol Mall or enjoying an NFL game at the bar, attendees gathered for serious and productive conversation the day before the opening of the PDA Regulatory Conference 2025, leaving panelists and the moderator in awe of their dedication.
The workshop opened with an introduction to the Capital Area Chapter and its mission by chapter president Martin Jenkins, Senior Consultant, Circle MJ Consulting. He said one of the goals of the chapter is to bridge the gap between professional and personal well-being in the pharmaceutical industry, a field known for its high stress. The chapter also focuses on empowering professionals through innovation and networking.
Jenkins stressed that all proceeds from the roundtable were to fund the “PDA Jette Christensen Early Career Professional Grant” to support the next generation of pharma leaders.
He then handed the meeting over to Glenn Wright, President and CEO of PDA, who welcomed attendees and highlighted the importance of the event, which featured a top-tier panel of experts from industry and regulatory backgrounds. Panelists each brought decades of experience in quality, compliance and regulatory affairs. It included:
- Former U.S. FDA official Tom Cosgrove, Partner, Covington & Burling LLP
- PDA Chair Anil Sawant, SVP Global Quality Compliance, Merck
- Ghada Haddad, Head of Global Quality Systems and Processes, Kite Pharma
- Former FDA official Stelios Tsinontides, VP QMS, Transformation and External Advocacy, Merck
The FDA Complete Response Letter Landscape
The central topic was the FDA’s Complete Response Letters (CRL), which are issued when a drug application is not approved. CRLs have become increasingly common, with facility issues being a significant driver. The impact of a CRL is substantial: it delays patient access to new medicines, can financially devastate small companies and often requires a full resubmission, extending timelines by a year or more.
Historically, CRLs were not publicly available, making it difficult for industry to learn from them. Recent improvements in transparency allow for better understanding, though much information remains redacted.
CRL Trends and Data
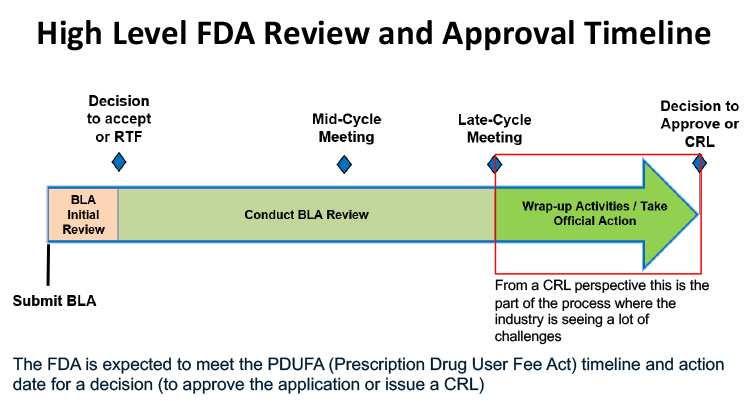
Data presented showed a marked increase in CRLs from 2020 through 2024, with facility-related deficiencies as a major cause. The approval process was outlined: after submission, applications undergo initial review, mid-cycle and late-cycle meetings and, finally, either approval or a CRL. Late-cycle issues—such as last-minute inspections or document requests—were identified as problematic, often leaving insufficient time for resolution.
Industry Challenges
Panelists discussed several challenges:
- Timing of Pre-Approval Inspections: Late inspections can jeopardize approvals.
- Late Document Requests: Last-minute requests strain resources and delay responses.
- Increasing GMP Expectations: Evolving standards, sometimes inconsistently applied, create uncertainty.
- Lack of Visibility: Manufacturing-related CRLs are often opaque, making it hard for sponsors to anticipate or address issues.
- CDMO/CMO Dilemmas: When a contract facility receives a CRL, it can affect multiple sponsors, but confidentiality agreements often prevent sharing of critical information.
Regulatory Perspectives
Cosgrove provided insight into FDA’s evolving approach. He noted that the main regulatory tool has shifted from warning letters to refusal to approve applications, which has immediate and significant consequences. He highlighted inconsistencies in how GMP issues are handled between different FDA offices, sometimes resulting in CRLs even when surveillance inspections classify a facility as acceptable.
A key issue is the use of “Potential OAI” (POAI) alerts—flags indicating possible compliance problems. Increasingly, CRLs are issued based on POAI alerts, even before a final compliance decision is made. This can lead to situations where a facility is later deemed compliant, but the CRL stands, forcing resubmission.
Cosgrove argued for consistent standards: a GMP issue should only justify a CRL if it is significant enough to warrant an Official Action Indicated (OAI) classification. He also emphasized the need for transparency and timely communication, especially for contract manufacturing organizations (CMO) serving multiple clients.
FDA Panel Response
Tsinontides and other FDA alumni clarified that CRL decisions are not taken lightly and involve careful review of inspection findings. They acknowledged the challenge of confidentiality: FDA is legally restricted from sharing facility-specific information with sponsors other than the facility owner. This places the onus on industry to ensure quality agreements with CMOs include provisions for sharing inspection outcomes.
Panelists agreed that quality agreements are often difficult to negotiate and enforce, especially when there is disagreement about whether an observation impacts a particular product. The lack of clarity can lead to uncertainty and panic among sponsors.
Scientific and Policy Issues
The discussion turned to scientific standards, particularly in environmental monitoring and aseptic processing. Panelists noted that evolving standards are not always reflected in guidance documents, leading to surprises during inspections. They called for more frequent updates to guidance and better training for inspectors, emphasizing that companies should not learn about new standards for the first time during an inspection.
The concept of “materiality”—what constitutes a significant enough issue to justify a CRL—was debated. Panelists advocated for accepting proposed corrective actions in cases of ambiguity, especially when scientific consensus is lacking.
Transparency and Communication
An audience member raised the issue of thresholds for CRLs and the need for FDA to publish more detailed information about the causes of CRLs. Panelists agreed, noting that real-time feedback and detailed post-action letters would help industry learn from others’ experiences and improve compliance.
The panel also discussed the importance of risk–benefit analysis, especially for breakthrough drugs addressing unmet medical needs. In such cases, the agency may weigh risks differently, considering the broader impact on patient access.
Practical Advice
Panelists offered practical advice for working with CMOs:
- Negotiate robust quality agreements that require sharing of inspection findings.
- Ensure clarity on what constitutes an objectionable condition and how it will be communicated.
- Prepare for evolving standards by investing in ongoing training and staying abreast of regulatory trends.
- Advocate for more transparency from FDA and industry collaboration to address systemic issues.
Conclusion
The workshop concluded with a call for continued dialogue between FDA and industry, emphasizing the need for transparency, consistency and collaboration. The panelists were thanked for their insights, and the importance of supporting early-career professionals was reiterated.


