PDA Hosts Industry Focused Roundtable on Speeding Innovation through Global Regulatory Convergence
 One of the things I like most about PDA is the ability to get members together to discuss important topics and have them share
their views with each other. That was on full display at the PDA Week Roundtable on Global Regulatory Convergence held in Long Beach, California, on March 25th, 2024.
One of the things I like most about PDA is the ability to get members together to discuss important topics and have them share
their views with each other. That was on full display at the PDA Week Roundtable on Global Regulatory Convergence held in Long Beach, California, on March 25th, 2024.
This topic, and the benefits it can provide, has been on the minds of the regulators and the industry for over 30 years and is getting renewed interest as digitalization continues to accelerate and shrink the divide between countries and cultures. This renewed interest made the timing perfect for the roundtable. A special thanks goes out to Amanda Bishop McFarland, Senior Quality Risk Management (QRM) and Microbiology Consultant at ValSource Inc., for helping me in facilitating the roundtable.
Roundtable Setup
The roundtable was focused on the industry’s perspective, and attendees were asked to assume, for the sake of the discussion, that anything is possible and to not restrict their ideas based on what they perceive as limitations today or how the current global regulatory process works.
Two focus areas were presented for the discussions, both addressing significant patient impact to help stress the urgency of the discussion. The focus areas were:
- The global process for new product submission, review and approval for novel, lifesaving products.
Lifesaving drug products were defined as products that treat conditions for which the patient has no other medical options and their condition is terminal.
- The global Post Approval Change (PAC) process requiring a submission, review and approval to implement manufacturing innovations that reduce drug shortages.
Manufacturing innovation to reduce drug shortage was defined as the implementation of new equipment with advanced automated process control (e.g., artificial intelligence [AI]/digital twin) that improves process performance (e.g., increased productivity and manufacturing consistency). In these cases, the changes being made to the manufacturing process must be submitted for review and approval by regulatory authorities.
 The two focus areas are both instrumental in accelerating the introduction of life-saving innovations across different world regions. The outcome of this workshop is relevant in different contexts as well, including, for
instance, expedited and global access to multiple pharmaceutical modalities needed to cure or prevent life-threatening diseases.
The two focus areas are both instrumental in accelerating the introduction of life-saving innovations across different world regions. The outcome of this workshop is relevant in different contexts as well, including, for
instance, expedited and global access to multiple pharmaceutical modalities needed to cure or prevent life-threatening diseases.
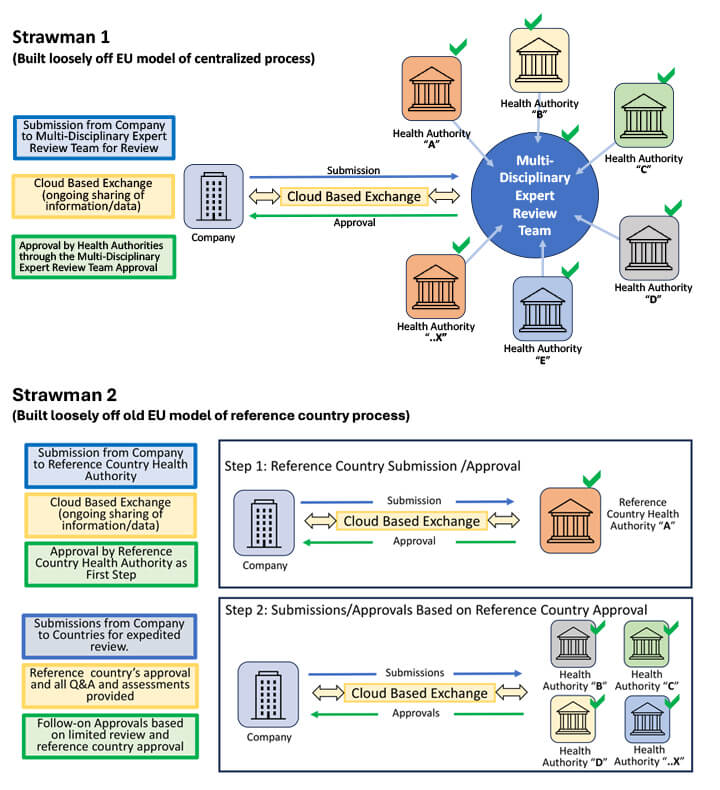
After setting the stage, the participants were challenged with the following: From an industry standpoint, if you had the power to establish a global convergent regulatory process for these focus areas that would speed innovation, what would it look like? Two high-level strawman models were provided and reviewed as part of the introduction to spark the discussion (link to Roundtable Opening Slide Deck).
There were just examples based loosely on EU models with the addition of a digitalization aspect (a cloud-based exchange for information sharing). Attendees were encouraged to bring up their own ideas as well as challenges and concerns. There was a very high level of engagement in the discussion, and overwhelmingly, attendees expressed that from an industry perspective, global regulatory convergence is an area where significant further progress is needed and where the rate of that convergence needs to be greatly accelerated. The benefits to both the patients and society were clear.
Roundtable Discussion Summary Highlights
Listed below are points captured from the roundtable discussion.
- An industry wide initiative is needed in this area and the industry needs to be:
- Speaking with one voice
- Leading rather than lagging
- More proactive in stating specific industry wants and needs
- Clearly stating why the industry needs it and the capacity the industry has to support it
- Regarding Strawman 1
- Strategically, if there is a desire to go fast to be ahead of the competition, in the current model, the sponsor may choose to specify which country reviews the application in order to get approvals faster; we need to think about how this is addressed in the new model.
- Questions to consider:
- Will recognition agreements be required for all countries/regions involved? If so, those would need to be in place as a precursor to achieve the desired state.
- Will more than just recognition agreements be needed? The level of collaberation and sharing of information globally requires changes to current laws and possibly even new treaties to be put in place.
- Regarding Strawman 2
- What would stop the follow-on reviewers from slowing down the process?
- Trust would need to be established and would be an enabler.
- The number of individuals involved in the follow-on process may be an important factor. Fewer may be better in this case as long as they are qualified.
- Questions to consider:
- How to address the language barrier if only one submission is to be sent out globally. Can AI be leveraged to help with translation and question interpretation?
- Regarding Strawman 1 and 2
- Technology differences: What should we do about technology differences (e.g., different platforms in different countries or lack of technology infrastructure to support the cloud exchange)?
- To make it work, the shared technology/system would have to be low-cost or shared-cost.
- Application of platform knowledge and the use of common language across all production lifecycle phases globally would be needed. This is an important foundational step in a shared global regulatory process (i.e., convergence), and it would most likely require some type of new global regulation or guidance.
- Risk appetite: For the various models, identifying and understanding the risks associated with each model and the application of countermeasures for those risks will be important. Since many are vested in the current process with its complexities, moving to a streamlined convergent process would require a true desire to change and would require overcoming the inherent inertia present.
- Political and economic considerations: Changes that drive efficiency may potentially impact revenue generation from submission/user fees as well as agency and industry staffing levels.
- Harmonization of requirements: Underpinning the process would be the need for harmonization of requirements – a single set of global requirements.
- Public/citizen Engagement
- To make such a change, will public/citizen action be required to move forward based on the anticipated inertia? – What did we learn from COVID?
- 1 global submission
- 1 global inspection (pre-approval inspection)
- 1 global approval
- Risk Aversion
- A shake-up in the business risk aversion while keeping the risk to the patient in the forefront (i.e., separate business risk and patient risk).
- A more rapid adoption of advanced modeling/digital solutions to help de-risk decision-making for product and process development throughout the lifecycle.
- A resolution on how quality management maturity (QMM) feeds into this process and speeds the implementation of innovation (i.e., QMM as an enabler).
- QMM measurement – i.e., QMM and performance scores could qualify a company/site for expedited access to global regulatory convergent processes and greater allowance for management of change internally (e.g., no reporting globally).
- Regulatory Relief
- Good and bad Actors
- Today, the good actors are not seeing the relief expected from having developed and implemented strong systems to ensure GXP compliance and high-quality regulatory submissions to align with the regulator’s requests/expectations.
- The bad actors are holding the industry back – the chain is only as strong as its weakest link. How can we differentiate the good from the bad (have two chains)?
- Without this differentiation, the industry is programmed to follow the rules with a culture that does not actively promote change. How do we change this so the industry can unlearn this behavior that stifles innovation?
- Post-Approval Changes (PAC)
- If the quality system is robust and the regulators trust the companies to follow it, then maybe 80% of changes (PAC) can be addressed/managed through the quality system, and only 20% would require submissions/inspections.
- In the future, could a company be qualified to do this and get on the “good list” for reduced submissions/inspections?
- Companies need to step up and get their houses in order to deserve an expedited process if available.
- PIC/s is a good example of collaboration (convergence) that helps facilitate drug manufacturing. It includes an actual checklist to follow to enure people are following the right processes.
- Maintaining the Focus on Science
- Harmonization – Q&A Process
- On the topic of the global multiple review model currently in place, if 100 questions are received from multiple authorities and 90 of them are very much the same for all regulators, can the remaining 10 related to regional differences then be the focus to speed up the approval process? It has been discussed in the European Union, but no real progress has been made yet.
- Ideally, a harmonized process would yield a single global list of questions.
- Ambiguity: As products become more sophisticated, divergent ambiguous regulations hinder the current process and limit the speed at which things happen (the process actually gets slower). How can this be addressed?
- Sustainability: Sustainability goals may prompt urgent PACs (e.g., modify the process to mitigate environmental impact). Is this another high-urgency category?
If we think about the desired state and a global convergent process, the goal should be:
An aversion to risk is a huge barrier to change both internally at a company level and externally at a regulatory agency level. To be successful, there will need to be the following:
How does the industry align and gain one voice to approach the regulators for relief (change) to move the convergence discussion forward?
For any changes to speed up the global regulatory processes through convergence, science must always remain the primary focus, and convergent processes must be designed to ensure this. The reality is that some bad actors will most likely try to cut corners, and the convergent global regulatory process will need to be able to detect these bad players.
Next Steps
There was a universal agreement as the roundtable wrapped up that more action needs to be taken to accelerate the topic of global regulatory convergence. It is a complex topic that the attendees felt the industry, regulators and, most importantly, patients could significantly benefit from. The atmosphere in the room was impatient regarding moving the topic forward, however, there was excitement at the prospect of a call to action. Based on the roundtable discussion, PDA will be announcing in the coming weeks the formation of a PDA Regulatory Convergence Forum to continue the dialogue and provide a space to foster ideas, initiatives and solutions focused on discovering the best path toward overcoming the challenges that face this incredibly important topic area. One of the first steps will be to continue working on developing a proposed high-level model from which details can be added.


