Per- and Polyfluoroalkyl Substances Ban Starting 2027
The European Chemicals Agency is proposing to restrict the use of per- and polyfluoroalkyl substances for industrial use starting in 2027.
Per- and polyfluoroalkyl substances (PFAS) are a broad class of synthetic chemicals whose typical properties, such as chemical inertness, high resistance to temperature and solvent, electrical inertness, corrosion protection, low coefficient of friction and non-flammability, make them appealing to several industry sectors. In addition, PFAS are used in a very broad range of applications:
- Textiles, upholstery, leather, apparel and carpets
- Food contact materials and packaging
- Cosmetics
- Heating, ventilation, air conditioning and refrigeration
- Medical devices and medicinal products
- Pharmaceutical manufacturing and products
- Transports
- Electronics, semiconductor and energy
- Construction products and lubricants
- Petroleum and mining

They contain alkyl groups in which all or many of the hydrogen atoms have been replaced with fluorine.
PFAS have been under public scrutiny over the years because they are labeled persistent organic pollutants that accumulate in the environment and pose risks to human health and living organisms (1). PFAS are categorized as polymers or non-polymer substances and have been used worldwide since the 1940s. Moreover, they were found to contaminate groundwater, surface water and soil. Specifically, long-chain PFAS are known to bioaccumulate and have already been banned (2, 3).
In March 2023, the European Chemicals Agency (ECHA) published a draft proposal, under Annex XV Restriction Report, for the restriction of PFAS. The restriction proposal addresses around 10,000 substances with the goal of preventing PFAS accumulation in the environment, food chain and effects on human health (3, 4). It has a significant impact across various industries, including the pharmaceutical sector. Once final, the restriction will be enforced under the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation as early as 2027. A restriction under REACH was considered the most effective tool to manage the risk of PFAS used in industrial processes and products.
The Annex XV restriction proposalis extensive, containing more than 200 pages with several annexes associated. Each annex provides additional information to the Annex XV sections and or subsections (e.g., manufacturing and use, information on hazard and risk and stakeholder information). In Annex XV, only two restriction options are mentioned:
- A full ban with no derogations and a transition period of 18 months.
- A full ban with use-specific time-limited derogation that involves an 18-month transition period, and either a five- or 12-year derogation period.
Potential derogation would require sufficiently strong evidence to justify its necessity.
Regarding the unlimited derogations, the intention is to cover special cases for practical reasons. The usage of PFAS as active substances in plant protection products, biocidal products and medicinal products is one of the few examples since these are covered under specific regulations.
Though time-limited derogations are mentioned in Annex XV, the pharmaceutical sector has been given limited consideration in the draft. As published by the European Federation of Pharmaceutical Industries and Associations (EFPIA), the consequences of the restriction proposal implementation would risk supply disruption and ultimately negatively affect the provision of medicines to patients (5, 6, 7). EFPIA has identified and categorized the areas, from drug development and commercial portfolio of medicinal products affected by the Annex XV restriction proposal, in the following groups (6, 7):
- Active pharmaceutical ingredients (APIs)
- Development products and APIs, including global programs
- Non-active ingredients (excipients)
- Starting materials and chemical intermediates
- Equipment and consumables
- Reagents, solvents, catalysts, auxiliaries in production and quality control
- Immediate packaging materials and sterile barriers and
- Drug delivery devices
A variety of APIs qualify in the broad Annex XV definition of PFAS. Examples include efavirenz (AIDS), mefloquine (malaria), fluoxetine (depression) and gemcitabine (cancer). Also, raw and starting materials, intermediates, auxiliaries, equipment and consumables required to manufacture pharmaceuticals and medical devices are in the scope of the restriction, directly affecting the manufacturing of medicinal products and devices. As a highly regulated sector undergoing rigorous registration and market authorization, pharmaceuticals aim for an exemption and derogation (5, 6).
Additional concerns with the Annex XV restriction proposal are related to the costs associated with the efforts to replace PFAS with viable alternatives and the high risk of unforeseen disruptions in the supply chains due to the associated economic impacts (7).
The Annex XV Restriction Report is the most complex substance restriction proposal in the European Union (EU) and is considered by the ECHA’s scientific committees for Risk Assessment Code (RAC) and Socio-Economic Analysis (SEAC). Further information may be submitted in the consultation on the Annex XV restriction proposal to enable ECHA’s committees to evaluate the justifications for any potential derogations. Therefore, stakeholders are invited to provide input until September 25, 2023, and all submitted restrictions for consideration can be found on the ECHA page.
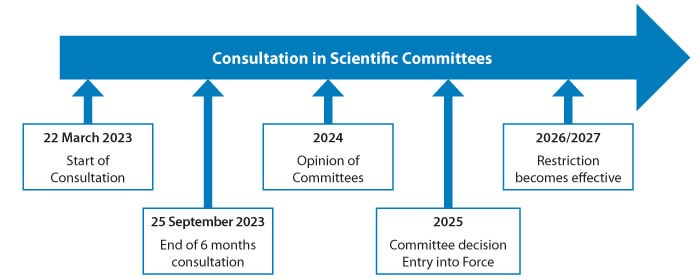 Figure 1 PFAS Restriction Proposal Timeline
Figure 1 PFAS Restriction Proposal Timeline
Afterward, ECHA’s RAC and SEAC committees will form an opinion based on the information in the restriction proposal and the comments submitted by the stakeholders. EU member states may provide advice to the committees as well. The European Commission will analyze the risks, benefits and costs associated with the RAC and SEAC opinions and the final decision is taken by the member states and the European Parliament. Figure 1 illustrates a timeline of the restriction proposal process regarding PFAS (8).
The industry has been forthcoming with its concerns and is actively following discussions and developments surrounding the PFAS restriction dossier in the EU. Stakeholders fully support regulatory action based on a comprehensive and consistent view of the available science. Currently, the ECHA consultation has received hundreds of submissions from stakeholders around the world.
References
- Stockholm Convention on Persistent Organic Pollutants - United States Department of State (2021a) U.S. Department of State. Available at: https://www.state.gov/key-topics-office-of-environmental-quality-and-transboundary-issues/stockholm-convention-on-persistent-organic-pollutants/ (Accessed: 28 August 2023).
- Blake, B.E. et al. (2020) ‘Evaluation of maternal, embryo, and placental effects in CD-1 mice following gestational exposure to perfluorooctanoic acid (PFOA) or hexafluoropropylene oxide dimer acid (HFPO-DA or genx)’, Environmental Health Perspectives, 128(2). doi:10.1289/ehp6233.
- Annex B, Annex to the ANNEX XV RESTRICTION REPORT, Version 2, 2023-03-22.
- ECHA, ANNEX XV RESTRICTION REPORT, Version 2, 2023-03-22.
- EFPIA, Position of the European Human Pharmaceutical and Animal Health Industry on the use of “per- and polyfluorinated alkyl substances” (PFAS) in Europe, in the light of a proposed Restriction under REACH as published, June 2023.
- EFPIA, (Representing European Pharmaceutical industry) and AnimalhealthEurope (representing Animal Health Industry) position on use and risk of “per- and polyfluorinated alkyl substances” (PFAS)1 in Europe, in the light of an emerging Restriction under REACH, January 2023.
- BioPhorum Operations Group Ltd, PFAS RESPONSE TEAM BioPhorum response to the Annex XV report on the proposal for universal PFAS restrictions, May 2023.
- The PFAS Restriction Proposal, Media Briefing Brussels, 2023-02-7.



 Ana Kuschel, PhD, as Principal Scientific Affairs of Europe, provide technical support relating to West Pharmaceutical Services’ packaging components and delivery systems for injectable drugs and healthcare products. In addition, she is also bridging scientific information through industry outreach.
Ana Kuschel, PhD, as Principal Scientific Affairs of Europe, provide technical support relating to West Pharmaceutical Services’ packaging components and delivery systems for injectable drugs and healthcare products. In addition, she is also bridging scientific information through industry outreach.