St. Gallen Seeks OpEx Info from Industry
[Editor’s Note: PDA is collaborating with the University of St. Gallen on a related project. Continue reading the PDA Letter for updates.]
Recently, the University of St. Gallen launched the Global Quality Benchmarking 2020 assessment with a goal of using to it to analyze operational data from around 2,000 manufacturing establishments in 52 countries for the University’s studies of pharmaceutical quality management practices and operational performance. This assessment was developed in collaboration with Dun & Bradstreet and funded by the U.S. FDA.
The short and user-friendly questionnaire was based on St. Gallen’s Benchmarking Performance Indicators and selected Enablers as these have been proven to best judge an overall system’s stability and performance. It asks questions about operational performance and quality maturity using 13 Key Performance Indicators (KPIs) in four dimensions, 18 Maturity Questions in three categories and five Contextual Factors. See Figure 1 for survey content.
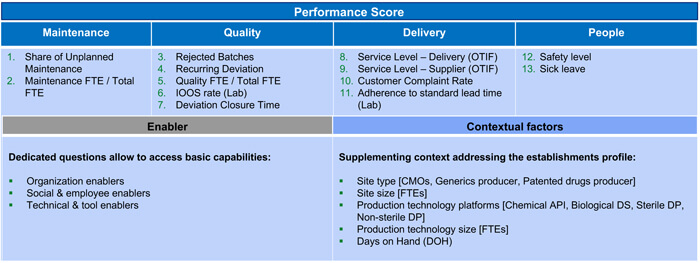 Figure 1 Baseline Pharmaceutical Quality Management Assessment
Figure 1 Baseline Pharmaceutical Quality Management Assessment
Developing this set of measures required extensive research, primarily using St. Gallen’s databases containing operational performance data from more than 380 manufacturing sites and 90 quality control labs. These databases were built over the last 15 years and summarize the outcomes of the full St. Gallen OPEX benchmarking programs. Based on the available data sets statistical exploration—such as correlation and regression analyses or t-tests—led to the subset of 13 metrics surrogating overall performance best. The research process is visualized in Figure 2. Additional validation comprising the direct comparison of an abbreviated overall performance score calculated based on the chosen measures only, and the full performance score used in the legacy benchmarking provided confidence from a system perspective.
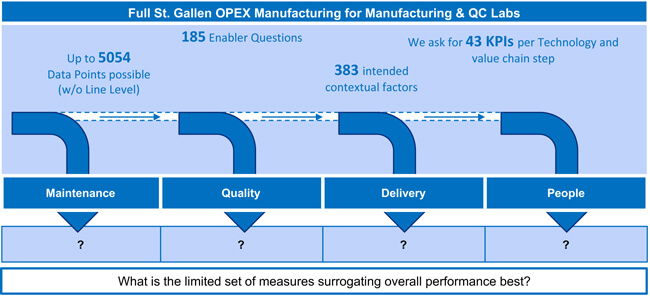 Figure 2 Assessment Development and Conceptualization
Figure 2 Assessment Development and Conceptualization
St. Gallen hopes the study will compare hundreds of establishments worldwide and rank the high performers among them. As a study with global reach, this ambitious initiative is made possible through funding provided by the FDA.
Conclusion
All pharmaceutical companies are invited to participate in this assessment free of charge. Every participating establishment will receive a customized benchmarking report. To participate, visit https://item.unisg.ch/global_quality_benchmarking. All data provided by participants will stay confidential.
References
- University of St. Gallen. «OPEX Benchmarking» www.opexbenchmarking.com (accessed May 28, 2020)



 Marten Ritz heads the Research Group Operational Excellence at the Institute of Technology Management, University of St. Gallen, Switzerland. In this role, he supports numerous pharmaceutical companies in continuously improving operations as well as launching/maintaining sufficient production systems, KPI strategies and quality metrics programs.
Marten Ritz heads the Research Group Operational Excellence at the Institute of Technology Management, University of St. Gallen, Switzerland. In this role, he supports numerous pharmaceutical companies in continuously improving operations as well as launching/maintaining sufficient production systems, KPI strategies and quality metrics programs.  Thomas Friedli is a Professor for Production Management at St. Gallen University in Switzerland. His main research interests are managing operational excellence, global production management and management of industrial services. He leads a team of 15 researchers who develop new management solutions for manufacturing companies in today’s business landscape.
Thomas Friedli is a Professor for Production Management at St. Gallen University in Switzerland. His main research interests are managing operational excellence, global production management and management of industrial services. He leads a team of 15 researchers who develop new management solutions for manufacturing companies in today’s business landscape.