Standing Guard

Comparing the Established LAL Assay to Current Alternative Endotoxin Detection Methods
Acceptance of the limulus amoebocyte lysate (LAL) test by the U.S. FDA required 15 years of side-by-side LAL and rabbit pyrogen testing to establish safety and efficacy of LAL as an endotoxin test. As alternative methods to LAL are being introduced to the market, what is the extent of the testing on real-world samples and how do the results compare across the range of critical characteristics, including specificity?
The Humble Beginnings of LAL
The rabbit pyrogen test first appeared in the U.S. Pharmacopeia (USP) in 1942 and became a standby in the parenteral industry. In 1964, Dr. Frederik Bang and Dr. Jack Levin famously discovered that LAL could detect the presence of endotoxins (1). This later prompted other researchers to recommend LAL as a potential alternative to the RPT for endotoxin testing, prompting worldwide interest in the unique reagent produced from the blood cells of the horseshoe crab (2).
As the rabbit pyrogen test was already established in the regulatory process, this required regulators and industry to confirm that the LAL test was more sensitive and more specific than the rabbit assay and to verify the absence of significant nonendotoxin pyrogens. In 1973, FDA published proposed manufacturing specifications for the production of LAL, allowing the industry to start gaining experience working with it. For large-volume parenteral (LVP) manufacturers, the end of batch (EOB) stage was considered the worst case and, hence, was subjected to rabbit testing. Recognizing the potential of LAL, in 1973 Baxter Travenol, the largest producer of medical devices and LVPs, decided to validate LAL as an endotoxin test in its global facilities. In reporting the results of their first study (143,196 LAL tests and 28,410 rabbits test on domestic water, IV solutions and medical device extracts), they concluded that (1) endotoxin was the only detectable pyrogen in parenteral products, (2) LAL was the more sensitive test, and (3) rabbit tests often gave equivocal results (3). Baxter Travenol’s compelling report to FDA in 1978 was critical to the Agency's decision to create an LAL test guideline.
In 1983, USP officially replaced the rabbit test with USP <85> Bacterial Endotoxins Test. The LAL test guideline was then issued in 1987 after 15 years of test experience and millions of LAL tests conducted globally.
Permission for limited application of the LAL test by the FDA in 1973 was intended to resolve two critical issues: endotoxin limits and specificity.
In order to develop and understand endotoxin limits properly, pharmaceutical and medical device companies conducted comparative studies that calibrated the biological fever response of rabbits to endotoxin concentrations measured by LAL. The Health Industry Manufacturing Association study in 1980 was a collaborative study that involved 12 laboratories. Using a single type of endotoxin, this study demonstrated that the threshold pyrogenic dose in rabbits was approximately 1.0 ng/kg (4). These data demonstrated that LAL reagent must detect a minimum of 0.1 ng/mL of an E. coli endotoxin (dosed in rabbits at 10 mL/kg).
The question of specificity (the ability to detect a range of endotoxins), which demonstrates the method is suitable for its intended use, was addressed by compelling data from Travenol. As a licensed LAL manufacturer with a single procedural specification for a global LAL testing program, they compiled an impressive amount of comparable LAL and rabbit test data. In a global study, a total of 213,352 LAL tests and 38,184 rabbit tests were conducted on raw materials and in-process samples (5). The rabbit tests were made on LAL failures and EOB tests. Most importantly, all of these tests were performed on materials contaminated with real-world, naturally occurring endotoxins, not with purified (LPS) standards. In all of Travenol's experience, there was not a single instance where the rabbit test failed and the LAL test yielded a passing result. These data and the nearly 45 years of experience of BET testing convincingly address the most serious concern for public health officials, namely, the test for specificity and false-negative results.
Recombinant Factor C
The current recombinant Factor C (rFC) assays are marketed as providing the latest advances while also using non-animal-derived products. Factor C is the first enzyme in the innate immune cascade of the horseshoe crab, detecting endotoxin by binding to it. rFC is a recombinant protein and is produced from the cloned sequence of the Factor C enzyme from the horseshoe crab species or subspecies. One rFC assay uses Factor C cDNA from Carcinoscorpius rotundicauda, the Singapore (mangrove) horseshoe crab, while the other rFC assay uses Factor C cDNA from a Tachypleus species (6–8). Thus, both rFC assays rely on the underlying assumption that the Factor C enzymes from all horseshoe crab genera (limulus, carcinoscoripus and tachypleus) will behave similarly to all of the proteins that are found within an aqueous amoebocyte extract.
All rFC assays are designed to provide results similar to compendial LAL assays and claim comparable assay times, sensitivities and required hands-on personnel resources. The average incubation period for rFC assays, however, is 60 minutes, significantly longer than some LAL technologies, which average 15 minutes from assay start to finish. Additionally, while tests have demonstrated that the sensitivity of rFC assays may be comparable to the LAL assay (0.005 EU/mL), results have never shown it to be superior to the compendial method. Finally, lengthy validation tests are required to obtain FDA approval for alternative endotoxin detection methods, necessitating significant additional resources. Above all, the decision to use alternative assays must be weighed against the potential for manufacturing or releasing products under a false-negative result due to the unproven specificity challenge.
Data from initial studies confirmed that both versions of rFC bind to and detect reference standard endotoxin and control standard endotoxin, which was hypothesized based on the similarities among Factor C enzymes from the three horseshoe crab species (7). A recent study presented data demonstrating the ability of rFC from C. rotundicauda to bind and detect purified LPS from various Gram-negative organisms, including Serratia marcescens, Klebsiella pneumoniae, Escherichia coli and Salmonella species (6).
Although a worthwhile beginning, the study does not address the ability of fluorescent rFC to detect real-world endotoxin contaminants seen in process streams.
A white paper, co-authored by a supplier of alternate endotoxin methods, compared two LAL reagents to three alternate endotoxin detection methods for their ability to detect naturally occurring endotoxins (9). Two of the alternate rFC methods were direct assays; the third method utilized a phage ligand, which is purportedly specific for bacterial endotoxins. The authors report that all methods demonstrated a 94.4% correlation. But what about the 5.6% that did not agree? Interestingly, a comparison of LAL and rFC from the same supplier revealed two water samples where rFC under-predicted endotoxin contamination by 80% and 91% respectively. In one instance, the endotoxin concentration measured by the phage ligand was approximately six times greater than both of the direct rFC methods. While glucan contamination and/or formulation differences were stated as potential reasons for discrepancies (the phage ligand is not affected by glucans), no attempt was made to ensure that specificity was thoroughly resolved. This is an unacceptable patient safety risk for many.
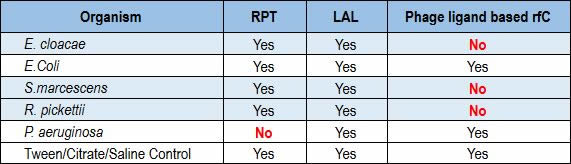
Recently, a team of researchers conducted a formal R&D protocol that investigated the performance of a ligand-based rFC test against the LAL and rabbit pyrogen tests. The phage ligand-based rFC tested negative for Enterobacter, Serratia and Ralstonia, while both the LAL and rabbit pyrogen tests were positive for all three (Table 1). In testing Pseudomonas aeruginosa, LAL showed a positive result where the rabbit pyrogen test showed a negative result (10).
Table 1 Correlation of the RPT, LAL and Ligand-Based rFC Assays in a Side-by-Side Test Involving Multiple Microbial Species (values in red indicate a discrepancy between the rabbit pyrogen test assay and the ligand-based rFC assay or the LAL assay)
Validation of Alternate Methods
Alternative methods can provide innovative solutions; however, the effort comes with inherent, costly challenges. Firms considering alternative assays should have a realistic understanding of the cost and time associated with validating these alternate assays as compared to LAL. Substantial resources are required to support a comprehensive and often lengthy validation strategy.
While the Ph. Eur. and USP provide guidance on key elements, such as precision, accuracy, linearity, detection limit, range and robustness, the critical issue associated with patient safety—namely specificity with naturally occurring endotoxins—is not provided. As a result, datasets comprising hundreds of data points must be compiled in order to assure safety, a potentially timely process.
In conclusion, while alternatives to LAL exist, the current, commercially available products should not be viewed as equivalent or a replacement for the FDA-approved LAL assay. Acceptance of the LAL test by the FDA required years of side-by-side testing of LAL and rabbit pyrogen test. That body of data facilitated the reliable interpretation of LAL assay results. The fact that, for the last 45 years, the pharma industry has routinely used LAL to support parenteral and medical device products stands as compelling testimony to the safety and efficacy of the LAL assay.
References
- Levin, J. and Bang, F. B. “The Role of Endotoxin in the Extracellular Coagulation of Limulus Blood.” Bulletin of the Johns Hopkins Hospital 115 (1964): 265–274.
- Cooper, J. F., Levin, J., and, Wagner, H. N. “New, Rapid, in-Vitro Test for Pyrogen in Short-Lived Radiopharmaceuticals.” Journal of Nuclear Medicine 11 (1970): 273–287
- Mascoli, C. C., and Weary, M. E. “Applications and Advantages of the Limulus Amebocyte Lysate (LAL) Pyrogen Test for Parenteral Injectable Products." Biomedical Applications of the Horseshoe Crab (Limulidae). (1979) Alan Liss, 150 Fifth Avenue, New York, NY 10011, 387–402.
- Dabbah, R., et al. “Pyrogenicity of E.coli 055:B5 endotoxin by the USP rabbit test -- a HIMA collaborative study.” Journal of the Parenteral Drug Association 34 (1980):212–216.
- Pearson, F.C., Weary, M.E., and Dabbah, R. “A Corporate Approach to In-Process and End-Product Testing With the LAL Assay for Endotoxin,” Endotoxins and Their Detection With the Limulus Amebocyte Lysate Test, 1982, Alan Liss, 150 Fifth Avenue, New York, New York, 10011.
- Ding, J. L., Navas, M. A., and Ho, B. “Molecular Cloning and Sequence Analysis of Factor C CDNA from the Singapore Horseshoe Crab, Carcinoscorpius Rotundicauda.” Molecular Marine Biology and Biotechnology 4 (1995): 90–103.
- Tan, N. S., Ho, B., and Ding, J. L. “High-Affinity LPS Binding Domain(s) in Recombinant Factor C of a Horseshoe Crab Neutralizes LPS-Induced Lethality.” Journal of the Federation of American Societies for Experimental Biology 14: (2000): 859–870.
- Abate, W., et al. “Evaluation of Recombinant Factor C Assay for the Detection of Divergent Lipopolysaccharide Structural Species and Comparison with Limulus Amebocyte Lysate-Based Assays and a Human Monocyte Activity Assay.” Journal of Medical Microbiology 66 (2017): 888–897.
- Reich, J., Heed, K., and Grallert, H. “Detection of naturally occurring bacterial endotoxins in water samples.” European Pharmaceutical Review 19 (2014): 67.
- Charles River Laboratories. Charles River Protocol Study 14-057, 2014.



 For the last 18 years, John Dubczak has been responsible for the production and technical operations of the Endosafe® brand of Microbial Solutions for Charles River Laboratories Microbial Solutions.
For the last 18 years, John Dubczak has been responsible for the production and technical operations of the Endosafe® brand of Microbial Solutions for Charles River Laboratories Microbial Solutions.