Storm Surrounds Clinical Trial Data in China
Recently, a “storm” has been brewing in China over the data integrity of clinical trial information. On July 22, 2015, the China FDA requested that the country’s pharmaceutical firms perform self-inspections of their China-based clinical trial sites. This self-inspection program, known as the “July 22 clinical trial data inspection storm,” has deeply impacted the healthcare business in China, almost on a historical scale. A large number of drug applications have been withdrawn or rejected, resulting in a significantly lower number of new drugs than anticipated.
The purpose of the self-inspection program was to give industry a chance to either confirm the integrity of the clinical trials and their associated data, or to terminate the clinical trials and/or retract submissions based on their trial data. CFDA would, thereby, lessen the inspection load for pharmaceutical companies, assuming industry would be sensible enough not to cheat in their self-inspections.
As this program nears its end and a more regular inspection program is expected to resume, it is a good time to review the outcome and lessons learned of this “inspection storm.” Although data integrity is emphasized by all regulatory agencies, none have put in place a program as stringent and wide-reaching as CFDA. In some ways, this agency has taken the lead on data integrity and international regulators may well adopt similar approaches. And these approaches may even trickle down to impact large-scale pharmaceutical manufacturing.
Data Integrity in Eye of Hurricane
The 2015 self-inspection program required firms to conduct self-inspections for clinical trials affecting 1,622 applications and submit their reports before Aug. 25, 2015 (1). If a company did not submit a report, that company had to withdraw the drug registration applications relying on this data. On Aug. 28, 2015, CFDA published a status update for the self-inspection program. Out of 1,622 applications, 67% of self-inspection reports were submitted and 20% (317) withdrawn; 193 applications (12%) were granted clinical trial waivers (2). [Note: These are the original numbers reported by CFDA; the authors are aware they do not add up.]
Then, in November 2015, CFDA published a document detailing the key points covered in these self-inspections (3). In the same month, CFDA rejected 11 registration applications from eight companies (4). Given the CFDA’s clear, firm, and unwavering stance on data integrity, a significant number of companies withdrew their existing registration applications.
Figure 1 Self-inspection Program Summary
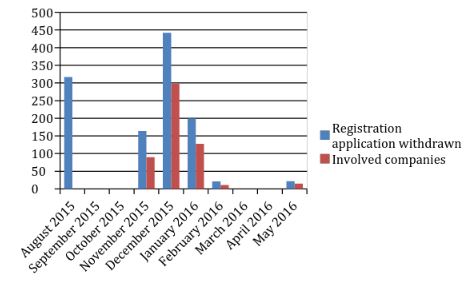
By the end of 2016, of the 1,622 pending applications, 1,165 were withdrawn voluntarily, 192 were granted a clinical trial waiver, and 30 were refused registration approval.
What were some of the findings from the self-inspection program? Typical inconsistencies included missing signatures (e.g., verification of data entries performed, but evidence unrecorded), incomplete archived records (e.g., missing original consent forms), and inadequate or absent access controls to computerized systems (e.g., analytical instrument data accessible by everyone through a shared set of credentials). In a few cases, gross misconduct was found, such as fictitious patients and fabricated clinical results.
What the CFDA Inspectors Found
Following the self-inspection program, the CFDA’S Center for Food and Drug Inspection (CFDI) conducted onsite inspections of the supporting data from approximately 200 clinical trial sites belonging to the remaining applications.
Though CFDA does not normally publish inspection reports, the authors became aware of the following issues identified during these inspections (in no particular order):
- Discrepancies between medication logs (sponsor shipping notes, hospital pharmacy logs, and patient files)
- Discrepancies between samples taken by an investigator or nurse and samples analyzed by the laboratory (e.g., there was no signed and dated sample transfer document, which is mandatory)
- Repeat analysis of samples contrary to trial protocol
- Incorrect age of trial subjects due to errors in date calculation (from the traditional Chinese calendar to the Gregorian calendar)
- Discrepancies between medical records and patient files with regards to medication not prescribed by the investigation site
Summary
The self-inspection program CFDA imposed on industry to verify the integrity of data from clinical trials and the subsequent agency inspections had the following desired effects:
- Retraction of a large number of applications based on unreliable or untrustworthy data
- Removal of a vast backlog of applications and subsequent shortening of review timelines
- Confirmation of CFDA’s approach that industry is capable of self-assessing, thereby lessening the load for agency reviewers
- Awareness by industry that Chinese regulators will enforce the law
- A vastly improved assurance for patients to receive medicines with supportive, trustworthy data
Since the CFDA’s intensified focus on data integrity of clinical trials began in late 2015, the regulatory agency and clinical trial sponsors have inspected all investigational sites/CROs involved or listed in applications. Following the self-inspection program CFDA announced in March 2016 plans to inspect clinical trial sites. This program has been ongoing and comes in waves (CFDA announced the ninth wave in January 2017) (5). And the focus on data integrity continues. It makes sense for pharmaceutical companies doing business in China to take note. Given the speed and success achieved, this program may well prove to be a blueprint for other regulatory agencies.
References
- Announcement of the State Food and Drug Administration on Carrying out Self-checking and Checking of Drug Clinical Trial Data (No. 117, 2015). Center for Food and Drug Inspection of CFDA. China FDA, July 22, 2015.
- State Food and Drug Administration on the drug clinical trial data self-examination of the notice (No. 169, 2015). Center for Food and Drug Inspection of CFDA, China FDA, August 28, 2015.
- Announcement of the State Administration of Food and Drug Administration on Releasing on-site Verification Points of Drug Clinical Trial Data (No. 228, 2015). Center for Food and Drug Inspection of CFDA. China FDA, November 10, 2015.
- Notice of the State Food and Drug Administration on 11 Enterprises Not Approving the Application for Drug Registration of 8 Enterprises (No. 229 of 2015). Center for Food and Drug Inspection of CFDA. China FDA, November 11, 2015.
- Bulletin on-site verification of drug clinical trial data (No. 9). Center for Food and Drug Inspection of CFDA. China FDA, Jan. 13, 2017



 Yingying Liu is a director with ECURAC, providing regulatory services for imported chemical, biologics and medical devices to register in China market. She has relocated to the US to complete a part-time regulatory certification course in Boston. She can be contacted at:
Yingying Liu is a director with ECURAC, providing regulatory services for imported chemical, biologics and medical devices to register in China market. She has relocated to the US to complete a part-time regulatory certification course in Boston. She can be contacted at:  Siegfried Schmitt is a principal consultant with PAREXEL, providing services to the regulated healthcare industry. He is a recognized subject matter expert in data integrity. He can be contacted at:
Siegfried Schmitt is a principal consultant with PAREXEL, providing services to the regulated healthcare industry. He is a recognized subject matter expert in data integrity. He can be contacted at: