Testing of the Future A Novel Mycoplasma Testing Technology to Increase Automation and Real-Time Release
Delivering safe products is essential, but ensuring rapid testing and release to patients is equally crucial, particularly for products with short expiry dates. Consequently, the pharmaceutical industry is diligently seeking rapid microbiology methods (RMM) to support rapid or even real-time release. The final goal is to transform the labs from manual and time-consuming testing into automated, digitalized laboratories, which eliminates the risk of human errors and enhances data integrity. However, it is still challenging to find solutions for “Microbiology Testing of the Future” that are available on the market and ready to implement.
During the 2023 PDA Pharmaceutical Microbiology Conference in Washington, D.C., October 2-4, a session on the first day entitled “Microbiology Testing of the Future” will reflect on the change that is already ongoing in many microbiology laboratories, not only giving new RMM but also concerning data management and building the competency of lab analysts.
A new, rapid and automated mycoplasma testing technology is the subject of one presentation in this session. Rapid polymerase chain testing (PCR)-based tests have emerged within the realm of mycoplasma testing. Health authorities accept them for testing of monoclonal antibody products as well as for advanced therapy medicinal products. Several PCR-based assays are commercially available, yet these typically still require extensive hands-on time for sample preparation and nucleic acid extraction, specialized personnel, and dedicated test areas. These methods have not prioritized automation and simplification. A novel PCR-based technology that overcomes these hurdles is the BioFire® FilmArray®. The biggest advantage of this technology over other PCR-based methods is the ease of use, comprising a nearly fully automated sample preparation and nucleic acid extraction followed by a nested multiplex PCR to amplify and detect mycoplasma RNA/DNA. Furthermore, results are available within just two hours.
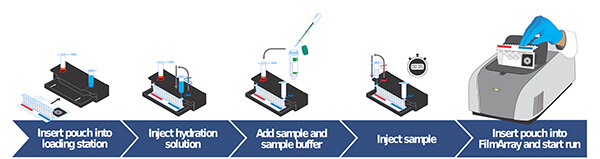
A fundamental aspect of this novel technology is the so-called “lab in a pouch" (see Figure 1), which encompasses distinct compartments for cell lysis DNA/RNA extraction, followed by successive multiplex PCR and detection in a closed system. This represents a remarkable stride in minimizing contamination.
Do not miss this event, where you will have the opportunity to hear about the generic method validation performed by Janssen, including a comparability study between the conventional mycoplasma assay and the Biofire® FilmArray® technology for a representative monoclonal antibody.
The validation approach, along with the implementation and regulatory submission strategy, will be shared with the audience. Janssen is the first company that filed the Biofire® FilmArray® technology for mycoplasma testing of a pharmaceutical product and received approval in multiple regions.
This serves as an example for driving innovation in a highly regulated environment, such as the pharmaceutical industry. To achieve success, early involvement of all stakeholders and close co-operation with the vendor are required. Understanding the risk concomitant with introducing new methods is essential to develop an appropriate validation, implementation and filing strategy. I am looking forward to seeing you at the 2023 PDA Pharmaceutical Microbiology Conference in Washington D.C., October 2-4!



 Heike Merget-Millitzer, PhD, is the Microbiology Centre of Excellence Lead within Janssen Inc. She has more than 25 years of work experience in aseptic manufacturing and testing of cell therapeutics and large molecules, as well
as in environmental monitoring in the pharmaceutical industry. She joined J&J in 2005, managing the manufacturing of clinical supplies, commercial products and new product introduction of sterile drug products before she managed the QC Microbiology
& Hygiene Department at Janssen Schaffhausen, Switzerland.
Heike Merget-Millitzer, PhD, is the Microbiology Centre of Excellence Lead within Janssen Inc. She has more than 25 years of work experience in aseptic manufacturing and testing of cell therapeutics and large molecules, as well
as in environmental monitoring in the pharmaceutical industry. She joined J&J in 2005, managing the manufacturing of clinical supplies, commercial products and new product introduction of sterile drug products before she managed the QC Microbiology
& Hygiene Department at Janssen Schaffhausen, Switzerland.