The Need for Manufacturing Speed Accelerating Vaccine Availability for Emergency Use Against Disease Threats

Advancements made in SARS-CoV-2 (COVID-19) vaccine development and recent mpox, avian influenza and Marburg virus outbreaks emphasize the need for technological innovations to speed up the creation of safe vaccines. The sooner protective vaccines can be developed and distributed to population, the sooner an outbreak can be contained. In 2021, the Coalition for Epidemic Preparedness Innovations (CEPI) launched its 2.0 strategy to meet this goal. Its mission: To “accelerate the development of vaccines and other biologic countermeasures against epidemic and pandemic threats so they can be accessible to all people in need.” The main goal is to prepare for future health crises, as discussed by Saville et al (1).
The Pfizer-BioNTech BNT 192b2 COVID-19 vaccine (Comirnaty®) marked a significant milestone by becoming the first vaccine to receive emergency use authorization (EUA) from a stringent regulatory authority in less than a year. From the release of the virus’ genetic sequence in January 2020, it took 326 days to obtain EUA status, by which time over 70 million human infections had been confirmed. To prepare better for future pandemics, an ambitious target has been set–“vaccines should be ready for initial authorization and manufacturing at scale within 100 days of recognition of a pandemic pathogen, when appropriate–a strategy known as the 100 Days Mission. A recent modeling study by Barnsley et al. estimated that the implementation of the 100 Days Mission in response to the COVID-19 pandemic could have saved over eight million lives, including many from Global South countries (2). (The Global South broadly comprises Africa, Latin America and the Caribbean, Asia (excluding Israel, Japan, and South Korea) and Oceania (excluding Australia and New Zealand) (3).)
To achieve the 100 Days Mission, CEPI 2.0 aims to reduce vaccine development timelines from under one year to just 100 Days. The 100 Days Mission is structured around three fundamental pillars: Preparing for anticipated epidemic and pandemic threats, transforming the response to emerging novel threats and fostering enhanced global collaboration. This requires a major shift in approach, including significant initial investment in vaccine development, innovative technologies, and creating a cohesive global network of vaccine distribution (e.g., a manufacturing network between CEPI, UNICEF and GAVI) to make vaccines accessible and affordable. Collaboration with regulatory authorities globally and other key stakeholders should facilitate the development of a framework that supports accelerated vaccine development and early deployment for future outbreaks. This framework could enable optimal use of platform data and preapproved documentation to prepare, develop and deploy vaccines to clinics efficiently.
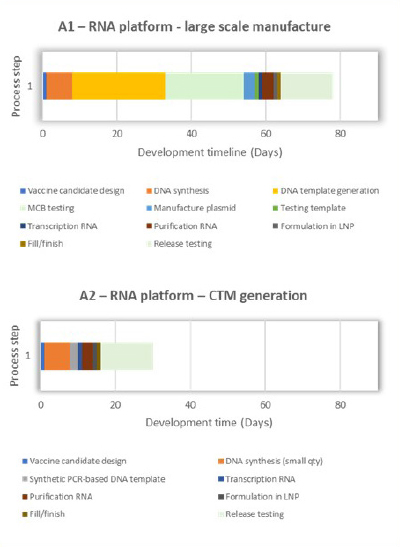
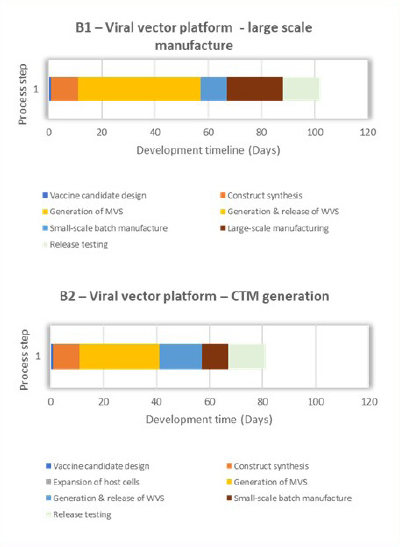
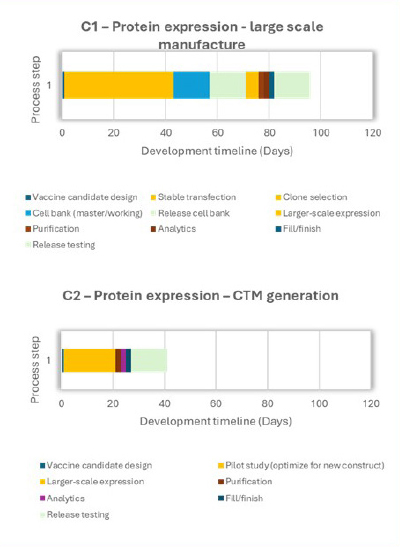
An extensive critical path analysis of COVID-19 vaccine-development timelines towards vaccine availability identified manufacturing, specifically chemistry manufacturing and controls (CMC), as being on the critical path for making a new vaccine for first-in-human trials. The timelines for producing vaccines using proven and established methods, such as mRNA, protein and viral vectors, are shown in Figure 1. Common areas for timeline improvement for conventional, large-scale manufacture of RNA, viral vector and protein expression vaccine products were identified as cell-based process steps (orange and yellow blocks) and analytical release testing (light green blocks), as depicted in Figure 1: A1, B1 and C1. Figure 1: A2, B2 and C2 illustrate potential improvements that can be achieved through manufacturing technological innovations, which are discussed in the sections below. In 2025, CEPI will launch an analytical call for project proposals from the broader scientific community on analytical innovations that will accelerate vaccine release and characterization testing.
CEPI's Speed Call Seeks Innovations to Accelerate Vaccine Manufacturing
CEPI initiated a series of calls for proposals (CfPs) aimed at leveraging innovative technologies and manufacturing to enhance speed, scale and accessibility for vaccine development to support the 100 Days Mission aspirations. One of these CfPs, Innovations for vaccine manufacturability focused on speed, was launched in May 2023 in response to a pathogen outbreak with the objective of using innovations and technologies related to manufacturability to expedite the production of clinical trial material (CTM) for emergency use. This “Speed Call” aimed to identify innovative technological approaches that could speed up vaccine manufacturing while achieving a comparable product to that from the platform process. The four focus areas for process improvement were as follows:
- Platform process development, process optimization, standardization and acceleration (e.g., proven technology platforms like mRNA, viral vector, protein expression or other novel platforms in batch or continuous processing mode)
- Analytical technologies to speed up drug-substance or drug-product batch release (e.g., implementing existing batch-release assays, new technologies for vaccine product identification and potency tests, or establishing and implementing analytic reagents) and availability of master cell banks (MCB) or master viral stocks
- Innovations to accelerate cell-based manufacturing steps, including synthetic approaches (e.g., implementing cell-free manufacturing improvements for vaccine production)
- Other manufacturing innovations that can accelerate CTM availability (e.g., use of artificial intelligence (AI) in developing and controlling processes and facilitating product release and vaccine deployment)
 Technological innovations, such as the use of synthesized DNA templates for mRNA vaccine production, cell-free protein synthesis
and nonclonal cell lines for protein expression, can shorten vaccine manufacturing timelines as illustrated in Figure 1: A2, B2, C2, and will be discussed in later sections of this article. Platform processes can be optimized by operating
in continuous mode and analytical testing of drug substance or drug product can be expedited to reduce hold times between vaccine production and release. While rapid analytical release tests, particularly for sterility, adventitious agent detection
and potency assays, can speed up vaccine batch manufacturing, surrogate methods like antigen-agnostic potency testing can circumvent the need for developing time-critical antigen-specific reagents in new vaccine development. Additionally, the Speed
Call will aim to identify innovative technologies that can expedite analytical tests.
Technological innovations, such as the use of synthesized DNA templates for mRNA vaccine production, cell-free protein synthesis
and nonclonal cell lines for protein expression, can shorten vaccine manufacturing timelines as illustrated in Figure 1: A2, B2, C2, and will be discussed in later sections of this article. Platform processes can be optimized by operating
in continuous mode and analytical testing of drug substance or drug product can be expedited to reduce hold times between vaccine production and release. While rapid analytical release tests, particularly for sterility, adventitious agent detection
and potency assays, can speed up vaccine batch manufacturing, surrogate methods like antigen-agnostic potency testing can circumvent the need for developing time-critical antigen-specific reagents in new vaccine development. Additionally, the Speed
Call will aim to identify innovative technologies that can expedite analytical tests.
In response to the Speed Call, numerous proposals were submitted and subsequently reviewed, evaluated and selected, based on their alignment with CEPI’s strategic portfolio goals. The chosen projects aim to provide proof of concept for the proposed technological innovations in contrast to the standard industry methods for vaccine production. Data from comparability studies will include the physicochemical and immunogenic properties of vaccines produced, where applicable. The following sections provide a background of CMC challenges identified through critical path analysis and the projects funded by CEPI to expedite vaccine development and manufacturing.
Innovations to Expedite mRNA Vaccine Process Development
mRNA therapeutics have been under development for more than twenty years, and the COVID-19 pandemic accelerated efforts by researchers and manufacturers to create vaccines targeting SARS-CoV-2. The Pfizer-BioNTech Comirnaty® and Moderna Spikevax® vaccines demonstrated high efficacy and have heightened interest in the use of the mRNA platform for developing vaccines for other viruses and diseases. mRNA vaccine development involves a cell-free, enzymic process that begins with linearizing a DNA template that encodes a protein of interest. The DNA is transcribed to generate RNA molecules, which are then purified and formulated in lipid nanoparticles for cellular delivery. Once inside cells, the RNA molecules are translated into proteins, initiating an immune response to offer protection against infection.
Mapping of the mRNA process highlighted that cell-based steps, such as the bacterial production of plasmid DNA (pDNA)–the start material for mRNA vaccines–created bottlenecks in the rapid production of vaccines. The spike protein sequence (gene of interest) is introduced in a host bacterium (typically E. coli) for production to high yields, as pDNA, during fermentation. In addition to the growing need for pDNA, due to advancements in cell-and-gene-therapy sectors, its production is both time-consuming and expensive. Process steps, such as cell-line development, producer-clone selection, establishing MCB and setting up a working cell bank, creates a bottleneck in material supply. Furthermore, pDNA can be contaminated with host bacterial genomic DNA, endotoxins, proteins and RNAs, adding to the cost and timeline for purification, production and release-testing.
The field of DNA synthesis has grown significantly in recent decades with advances like human genome sequencing, offering new opportunities in research, development and commercialization (4). Production of synthetic DNA is necessary to meet the growing demand in various fields, including vaccines and therapeutics, as a research tool for chemistry, biology and material sciences. Synthetic DNA could become a suitable alternative to plasmid DNA for rapid vaccine development and production under EUA by regulatory authorities to manage disease at its origin. In addition to using synthetic DNA to enhance the development timelines of mRNA vaccines, CEPI has also invested in process improvements. These include exploring automated batch manufacturing, employing biocomputing and developing specialized processes designed to scale up production.
CEPI is funding a project with DNA Script to advance its capability to automate synthetic DNA template production, accelerate mRNA vaccine development timelines and improve vaccine readiness. The production of fully synthetic DNA and lower manufacturing costs will make the technology more accessible to vaccine developers in low- and middle-income regions. To assess the potential of synthetic DNA in vaccine development, CEPI is also supporting research at Afrigen Biologics to develop an mRNA vaccine for Rift Valley Fever. This vaccine will be produced using both synthetic and pDNA for comparison, and an automated batch-manufacturing timeline for mRNA drug-substance processing will be evaluated against standard batch-processing methods.
CEPI is funding work at BiologIC Technologies to explore the use of biocomputing and AI to accelerate vaccine development using the mRNA platform within an integrated continuous automated system as opposed to standard batch processing. Finally, on mRNA processes improvement, work at the University of Sheffield to establish proof-of-concept for the RNAbox™. It aims to reduce the cost of mRNA vaccine production and minimize the need for complex cold-chain storage and transportation infrastructure, thereby facilitating vaccine delivery to remote areas. The RNAbox™ will be designed to enable local manufacturing of mRNA vaccines at small production sites. Employing automated systems in these projects will allow the incorporation of analytical modules, like process analytic technologies (PAT), that are expected to facilitate real-time product quality monitoring and expedite vaccine release after production.
Innovations to Expedite Protein-Based Vaccine Process Development
Protein-based vaccines, which include purified proteins from a virus, recombinant proteins, virus-infected cells or virus-like particles (VLP), were among the majority of antiviral vaccines licensed for human use against SARS-CoV-2 (5). These vaccines typically require an adjuvant to induce a robust immune response. The Novavax NVX-CoV2373 vaccine (Covovax™, Nuvaxovid®) was among the first protein-based COVID-19 vaccines to receive conditional marketing authorization in the European Union in December 2021, significantly later than Pfizer-BioNTech’s mRNA and AstraZeneca/Oxford’s viral-vector vaccines.
Like the mRNA vaccine process, the mapping of protein-based vaccine manufacturing identified cell-based process steps as a significant bottleneck. Protein expression necessitates the development and optimization of a MCB. Mammalian cell lines are frequently employed to produce antigens that elicit an immune response when used in vaccines due to their ease of culture and high yield. However, developing and optimizing mammalian cell lines can take four to six months. The application of nonclonal cell lines–specifically stable pools and transient gene expression (TGE)–to produce biotherapeutics for toxicology studies and early-phase clinical trials have been documented (6).
During the COVID-19 pandemic, pharmaceutical companies reportedly used nonclonal stable Chinese hamster ovary (CHO) pools from well-characterized cell lines to produce monoclonal antibodies for preclinical safety studies, formulation studies and Phase I clinical trial material supply. This strategy allowed material availability within five months compared to the 12 to 14 months typically needed for monoclonal cell lines and was accepted by the FDA for emergency use (7). TGE cell lines were also employed to develop antibodies for biotherapeutics production; however, they were not used for clinical applications (8, 9). To assess the feasibility of using nonclonal cells for expedited vaccine production, CEPI is collaborating with scientists at the National Research Council, Canada on a preclinical proof-of-concept project aimed at developing a platform technology for vaccine clinical-material production using stable pools and TGE cell lines.
Cell-free protein synthesis (CFPS), also known as in vitro protein synthesis, is an innovative technology that facilitates protein transcription and translation without the need for cellular growth. Since its development in the 1950s, cell-free systems have seen significant advancements and are now used on an industrial scale for producing therapeutics such as antibodies and cytokines (10). These systems comprise crude cell lysates that are clarified by removing extracts such as cellular debris and chromosomal DNA, leaving only the essential factors for protein synthesis. The cell lysate can be frozen or freeze-dried and reconstituted by thawing or rehydrating when required. Protein synthesis is initiated by adding a DNA template with the gene of interest and other necessary reaction components. CFPS could become a promising alternative to cellular protein expression, since cell lysate can be produced and stored until needed for vaccine development, once the DNA template encoding a pathogen’s surface protein sequence is available. This can, in turn, significantly reduce the timeline for vaccine development by eliminating the lengthy process of cell-line development that usually takes four to six months and facilitate the rapid production of protein antigens needed for vaccine development during a virus outbreak.
While CFPS technology requires further refinement, CEPI is funding feasibility work at LenioBio for the application of their plant-based ALiCE® technology; this might enable the production of vaccine clinical material in 20 to 40 days as compared to an approved cell-based vaccine development method. The feasibility work will be carried out using synthetic DNA, which can potentially overcome the bottleneck in pDNA availability. CEPI is also funding feasibility work with at the Centre for Process Innovation (CPI) for the development of a self-contained manufacturing on the go (MANGO) device that aims to automate the process for VLP manufacturing. The MANGO-device concept developed at the University of Toronto will negate the need for cell cloning and could reduce the process for VLP manufacturing from seven to nine days to a single day. CPI will be working collaboratively on this project with a multinational consortium of partners to accelerate vaccine manufacture.
CEPI is also supporting Algenex in a project to validate the feasibility of the innovative baculovirus/insect pupae (CrisBio®) platform technology for human vaccine development. Over the past three decades, the baculovirus-vector expression system (BEVS), along with insect cells, has been extensively used for producing recombinant proteins, including vaccines. The traditional BEVS approach involves bioreactors that need optimization and scaling for commercial production. In contrast, the CrisBio® technology leverages specially bred chrysalises as living biofactories capable of generating large quantities of viral antigens more quickly than bioreactor methods and has already been successfully used in certain animal vaccines.
Innovations to Expedite Viral Vector Vaccine Process Development
Adenoviruses are non-enveloped double-stranded DNA viruses that cause mild and self-limiting human infections, like respiratory tract infections. Recently, adenoviruses have been used as carriers for drug and gene delivery and vaccine development, due to their inability to integrate to the human genome. To inhibit viral replication, the E1 and E3 viral genes are deleted and replaced with a gene encoding the desired antigen (11). This prevents the virus from replicating its genome post-delivery, focusing instead on producing the desired antigen.
Adenovirus-vectored vaccines are known to elicit strong antigenic responses, providing effective immunity. Notable examples include AstraZeneca/Oxford’s ChAdOx1 nCoV-19 (Vaxzevria®) and Johnson & Johnson’s Ad26.COV2-S (Jcovden™, previously as Janssen™ vaccine (12). These vaccines received EUA from the Medicines and Healthcare products Regulatory Agency in December 2020 and from the U.S. Food and Drug Association in January 2021. The AstraZeneca/Oxford vaccine was particularly affordable for low- and middle-income countries and could be stored at 2 °C to 8 °C, making it suitable for global distribution. By accounting for a third of all released vaccine doses in 2021, over three billion doses were distributed worldwide, until the vaccine’s planned withdrawal from sale in May 2024.
Like protein-based vaccines, generating a master and working viral stock in cell-based processes has been identified as a major hurdle in accelerating vaccine development. CEPI is funding strategic partnership work at the University of Oxford to accelerate the development of safe, effective and globally accessible vaccines against the threat of unknown pathogens with potential to cause pandemics. While Joe et al. have proposed ways to optimize viral seed production (13), CEPI’s partnership with the University of Oxford could pave the way for rapid response vaccine platforms in line with the CEPI 100 Days Mission.
Innovations to Expedite Analytical Methods and Technologies for Product Batch Release
Analytical technologies are on the critical path for the development and manufacturing of vaccines, given the long release timelines typically required for such tests as product sterility, detection of adventitious agents and potency assessments. Furthermore, the development of antigen-specific reagents and standards, particularly for unknown diseases, is time-consuming. Consequently, new rapid methods that do not depend on antigen-specific antibodies or other reagents for the accurate assessment of a vaccine’s potency will be necessary to enable quick and reliable vaccine development in response to an epidemic or pandemic outbreak.
The development of robust analytical methods is essential to ensuring the safety, efficacy and quality of vaccines and facilitating faster vaccine development. Compendial methods, such as sterility-testing, involve culturing product samples for up to 14 days to detect viable microorganisms. However, rapid sterility tests use technologies like bioluminescence, colorimetric and autofluorescence to identify microbial presence through the breakdown of adenosine-triphosphate, a primary energy source for cellular processes including growth. These techniques enable quicker detection of microbial contamination, generate standardized and automated readouts (eliminating analyst subjectivity) and enhance data integrity by removing the need for contemporaneous verification of results (14).
Analytical tools for confirming the identity of antigens vary with the type of vaccines, and molecular biology techniques such as reverse transcription polymerase chain reaction are increasingly used to confirm the identity of vaccine antigens, while sequencing is used to confirm the identity of mRNA, DNA and viral-vector vaccines. Enzyme-linked immunoassays use antigen-specific or pathogen-specific antibodies to determine identity, while technologies such as mass spectrometry-mapping assesses post-modification to a vaccine. Vaccines made using cell-based systems may be contaminated by host cell proteins, endotoxins and adventitious viruses. Endotoxins are usually detected with assays like the limulus amebocyte lysate test, while advanced techniques like high-through-put sequencing are being developed to detect adventitious viruses.
Key analytical trends that are likely to influence rapid vaccine development include the use of in/at/online PAT during production to reduce manufacturing testing requirements and the design of modular analytical tools that can be adapted and readily validated for rapid deployment. The use of multiplexed assay formats, where different assays are integrated to identify various antigen targets, are on the increase. New technology platforms such as bioinformatics and in-silico modeling are in development to speed up the design and validation of vaccine antigens. In-vitro methods tend to be preferred for batch release as they are more precise and robust than in-vivo assays and generate quicker results. Potency assays measure the functional integrity of an antigen and are intended to ensure that the vaccine retains its ability to stimulate a desired immune response. New potency assays will need to be implemented.
Analytical techniques and technologies for the discovery and process developments have varying requirements. The former need to be adaptable and capable of high-throughput functionality, while quality control tools for single-batch manufacturing do not necessitate high throughput. Quality control methods should be fixed, reliable and, preferably, automated to minimize the likelihood of human error. The implementation of these technological innovations will help to expedite vaccine product batch release.
Discussion and Conclusion
 The COVID-19 pandemic highlighted the challenges viral pathogens place on global society, emphasizing the need for better preparedness
among vaccine developers, regulators and health authorities for future epidemics and pandemics. Various vaccines were developed using different platforms, such as nucleic acid-based, adenovirus-based, protein-based and inactivated vaccines, with many
receiving approval to prevent serious COVID-19 diseases and death (11). Rapid development, scalable manufacturing and equitable access to vaccines are essential since public vaccination is the most effective strategy to control
the morbidity and mortality caused by the spread of viral pathogens. The CEPI 100 Days Mission aims to prevent future pandemics by promoting a significant change in strategy and utilizing technological advancements to expedite the development of emergency-use
vaccines for emerging pathogens.
The COVID-19 pandemic highlighted the challenges viral pathogens place on global society, emphasizing the need for better preparedness
among vaccine developers, regulators and health authorities for future epidemics and pandemics. Various vaccines were developed using different platforms, such as nucleic acid-based, adenovirus-based, protein-based and inactivated vaccines, with many
receiving approval to prevent serious COVID-19 diseases and death (11). Rapid development, scalable manufacturing and equitable access to vaccines are essential since public vaccination is the most effective strategy to control
the morbidity and mortality caused by the spread of viral pathogens. The CEPI 100 Days Mission aims to prevent future pandemics by promoting a significant change in strategy and utilizing technological advancements to expedite the development of emergency-use
vaccines for emerging pathogens.
This is an overview of proof-of-concept innovative technology projects funded by CEPI that have the potential to significantly reduce the timeline for vaccine development, in support of the 100 Days Mission. These projects will explore a variety of approaches–using validated technology platform process development and optimization in continuous mode, utilizing synthetic biology methods for DNA template synthesis for use in mRNA vaccine development and protein antigen production, employing characterized nonclonal cells, investigating the baculovirus/insect pupae protein expression system, and CFPS–to enhance the speed of vaccine manufacturability.
These projects aim to generate comparability data to demonstrate that vaccine material produced using these innovative technologies is equivalent to that produced using approved platform technologies for vaccine development. The comparability data will be derived from evaluating physicochemical and preclinical immunogenicity results to confirm that accelerated processes yield products equivalent to those produced using current technologies.
Accelerating analytical testing of drug substance and product can reduce the delays typically experienced between vaccine batch manufacture and release. Surrogate methods like antigen agnostic potency testing can be used to circumvent the need for developing time-critical antigen-specific reagents in new vaccine development. CEPI will be open to innovations from a wide range of sectors and will invest toward the implementation of these technologies for vaccine development and manufacturing.
References
- Saville, M., Cramer, J. P., Downham, M., Hacker, A., Lurie, N., et al. (2022). Delivering pandemic vaccines in 100 days - what will it take? N Engl J Med 2022;387:e3. doi:10.1056/NEJMp2202669
- Barnsley, M., Mesa, D. O., Hogan, A. B., Winskill, P., Torkelson, A. A., et al. (2024). Impact of the 100 days mission for vaccines on COVID-19: A mathematical modelling study. The Lancet, Vol 12 (11), E1764-E1774. https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(24)00286-9/fulltext
- United Nations Trade & Development. “Classifications: Country Classifications,” UNTAD Data Hub. https://unctadstat.unctad.org/EN/Classifications.html.
- Hoose, A., Vellacott, R., Storch, M., Freemont, P. S. (2023). DNA synthesis technologies to close the gene writing gap. Nature Reviews Chemistry, Vol. 7, pp. 144–161. https://www.nature.com/articles/s41570-022-00456-9#Tab1.
- Ghasemiyeh, M., Mohammadi-Samani, S., Firouzabadi, N., Dehshahri, A., Vazin, A (2021). A focused review on technologies, mechanisms, safety, and efficacy of available COVID-19 vaccines. International Immunopharmacology, Vol 100, Nov. 2021, 108162. https://www.sciencedirect.com/science/article/pii/S1567576921007980.
- Stuible, M., Frank van Lier, V. F., Croughan, M. S, Durocher, Y. (2018). Beyond preclinical research: production of CHO-derived biotherapeutics for toxicology and early-phase trials by transient gene expression or stable pools. Curr Opin in Chem Eng, Vol 22, pp. 145-151. https://www.sciencedirect.com/science/article/abs/pii/S2211339818300261.
- Agostinetto, R., Rossi, M., Dawson, J., Lim, A., Simoneau, M. H., et al., (2022). Rapid cGMP manufacturing of COVID‐19 monoclonal antibody using stable CHO cell pools. Biotechnol Bioeng. 2022 Feb; Vol 119(2), pp 663–666. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8652680/.
- Rodriguez-Conte, S., Inman, S., Lindo, V., Amery, A., Tan, A., et al. (2022). Suitability of transiently expressed antibodies for clinical studies: product quality consistency at different production scales. MAbs. 2022 Jan-Dec; Vol 14(1). https://pubmed.ncbi.nlm.nih.gov/35323099/
- Gonzalez-Rivera, J. C., Galvan, A., Ryder, T., Milman, M., Agarwal, K., et al. (2024). A high-titer scalable Chinese hamster ovary transient expression platform for production of biotherapeutics. Biotechnol Bioeng, 2024 Nov 121(11), pp. 3454-3470. https://pubmed.ncbi.nlm.nih.gov/39101569/.
- Varner, J. D., Vilkhovoy, M., Adhiknari, A., Vadhin, S. (2020). The Evolution of Cell Free Biomanufacturing. Processes 2020, Vol 8(6), p. 675. https://www.mdpi.com/2227-9717/8/6/675.
- Ghasemiyeh, P., Samani, S. M., Firouzabadi, N., Dehshahri, A., Vazin, A. (2021). A focused review on technologies, mechanisms, safety, and efficacy of available COVID-19 vaccines. International Immunopharmacology, Vol 100, Nov 2021, 108162. https://doi.org/10.1016/j.intimp.2021.108162
- European Medicines Agency. Jcovden (COVID-19 vaccine (Ad26.COV2-S [recombinant]) EMA/234937/2023. https://www.ema.europa.eu/en/documents/overview/jcovden-previously-covid-19-vaccine-janssen-epar-medicine-overview_en.pdf.
- Joe, C. C. D., Chopra, N., Nestola, P. et al. (2023). Rapid-response manufacturing of adenovirus-vectored vaccines. Nature Biotechnology, Vol 41, pp 314-316. https://doi.org/10.1038/s41587-023-01682-2.
- Deutschmann, S., Paul, M., Claassen-Willemse, M., Van den Berg, J., IJzerman-Boon, P., et al. (2023). Rapid Sterility Test Systems in the Pharmaceutical Industry: Applying a Structured Approach to Their Evaluation, Validation and Global Implementation. PDA J Pharm Sci Tech, Vol 77 (3), pp. 211-235. https://doi.org/10.5731/pdajpst.2021.012672.





