The Product Quality Leader Team Part II: Getting Ahead
 [Author Note: Part II of this series outlining AstraZeneca Biologics’ Product Quality Leader (PQL) Team shows how to employ a capability/skills matrix to both track and improve existing skillsets. Part I covered the creation of the PQL role.]
[Author Note: Part II of this series outlining AstraZeneca Biologics’ Product Quality Leader (PQL) Team shows how to employ a capability/skills matrix to both track and improve existing skillsets. Part I covered the creation of the PQL role.]
Soon after initiating the PQL role and building a team of PQLs, a capability/skills matrix was developed to help them succeed and grow. The primary objective was to raise awareness among PQL team members and management of the group’s current strengths and where gaps may exist.
Inspiration came from PDA’s experience using a capability/skills matrix to track the knowledge and levels of experience in key competencies of members in the PDA Biotechnology Advisory Board (BioAB). PDA wanted to see if the existing experience of BioAB members aligned with the Association’s long-term strategic plan. For most of its existence, BioAB has been capturing capabilities (knowledge/experience levels) for individual team members. This data is periodically updated and reviewed to align with PDA’s long-term vision and strategic plans. By focusing specifically on PDA’s planned areas of future growth, BioAB has deliberately used the available data on member capabilities to identify strengths and gaps. This has facilitated the establishment of appropriate priorities by anticipating which future deliverables were achievable and which were more challenging. Regarding gaps, PDA made strong efforts to recruit subject matters experts from across the industry and regulatory agencies to fill these gaps. Using this capability matrix has contributed to the long-term success of BioAB’s ability to support PDA.
PDA’s all-volunteer advisory boards direct and oversee topic-related activities and deliverables produced by all-volunteer task forces, interest groups and/or other PDA member-driven initiatives. Besides BioAB, volunteers serve on the Science Advisory and Regulatory Affairs and Quality Advisory Boards.
Transferring this concept to the PQL team has enabled the team to prioritize, based on gap/risk impact, and to develop extensive training and skill-building in areas where expertise is lacking.
Table 1 illustrates a simplified capability and skills matrix devised for members of the PQL team. Capability is scored and captured for (general) therapeutic areas and elements of manufacturing science of interest based on the established PQL review responsibilities and the current/expected diverse product portfolio. This is listed in the second column of the table (in light brown color). Column 3 (in light green) shows more specific PQL skills for direct tasks requiring review. While the more general capability areas were scored with three levels only (0%, 50%, 100%), a total of five levels (0%, 25%, 50%, 75%, and 100%) were used for specific review skills.
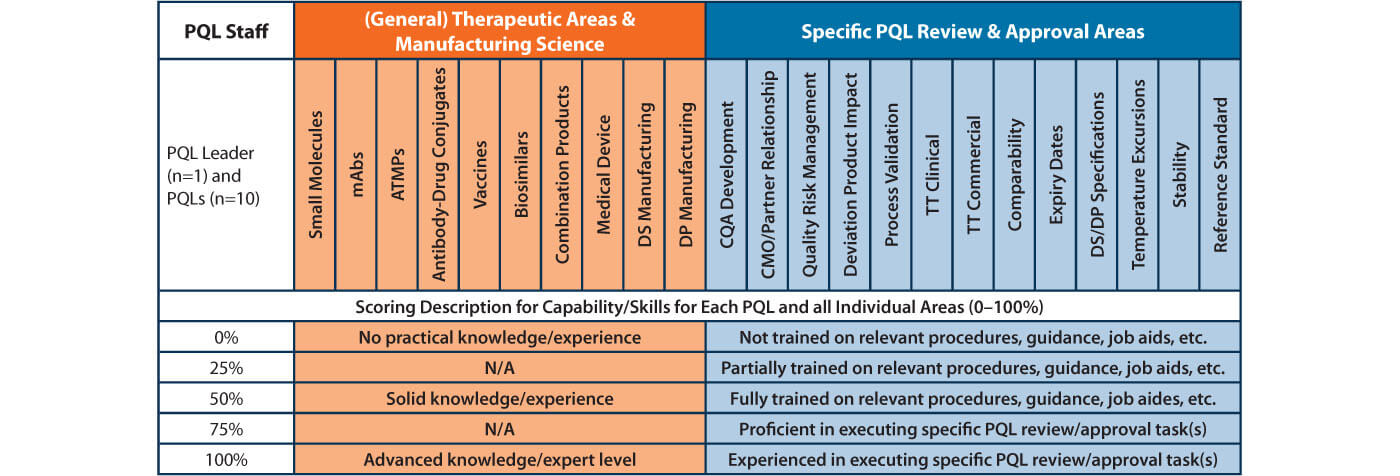
Table 1 Simplified Capability and Skills Matrix for PQL Team and Individual Members
Practical descriptions for all conditions were established, aligning with each capability or skill level. All PQLs used this information score themselves. These “raw data” scores were then used to capture truly representative data for each team member. Several major criticality factors, such as expected frequency of review events, based on current available product portfolio information, were then used as gap multiplication factors. Below is the list of criticality factors (calculations not shown here):
- Criticality (Gap) Factors for Consideration of Scores for Team and Individuals
- Number of expected reviews based on current product portfolio
- Proportion of products in current portfolio
- Assigned product priority
- Reliable internal consulting available
Figure 1 shows the initial overall team capability/skills scores for major review tasks in 2018. The sum of all “raw scores” of each team member represented an overall team score. Each sum was treated with the preestablished criticality factors and thresholds. The results clearly reveal that, within the general capability area, the overall experience level of the PQLs in manufacturing of monoclonal antibodies (mABs) is sufficient (> 50%), while the experience level within the advanced therapeutic medicinal products (ATMPs) is clearly a major capability gap given the existing team experience and criticality factors (only 7%, in red color). Within the specific skills, the review task of resolving temperature excursions for product in current distribution was identified as a major skill gap, since it was a new review task and these events happen relatively frequently as product is shipped and stored at many different global sites. With time and focused learning progressing, individual and team capability/skill levels are expected to increase faster in the specific review areas.
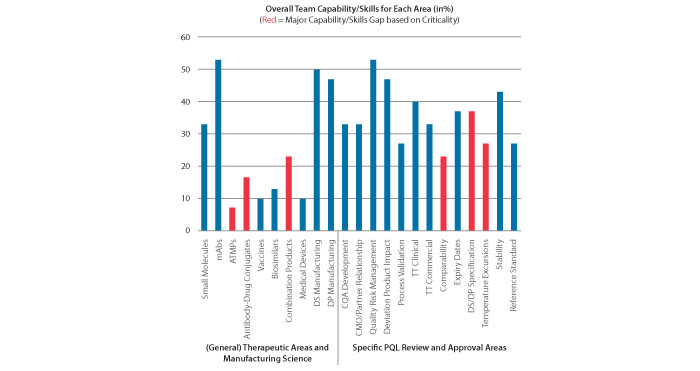
Figure 1 Initial Team Capability/Skills Scores (in %)
Periodic evaluations and updating of the capability/skill scores can be conducted relatively quickly and can now support PQL assignment changes whenever needed. In time, the expected increasing capability/skill scores for individuals and the overall team will be measured against short- and long-term function goals.
Management now has up-to-date knowledge of its function capabilities and can quickly adapt to expected and unexpected changes. For example, product portfolio and/or priority changes, such as a push for a highly accelerated product development following an agency-granted breakthrough therapy designation, can now be readily evaluated for impact on existing staff.
Part III will conclude this series by exploring another tool that further refined the PQL role: a Gemba Walk.



 Stephan Krause, PhD, is the head of AstraZeneca Product Quality Leader Group. He is a frequent PDA volunteer and current member of PDA’s Board of Directors.
Stephan Krause, PhD, is the head of AstraZeneca Product Quality Leader Group. He is a frequent PDA volunteer and current member of PDA’s Board of Directors. Mariam Khan has over ten years of biotech/ pharmaceutical industry experience in analytical sciences and quality working on biologics in clinical development. She is currently the PQL on cell therapies and various other monoclonal antibodies.
Mariam Khan has over ten years of biotech/ pharmaceutical industry experience in analytical sciences and quality working on biologics in clinical development. She is currently the PQL on cell therapies and various other monoclonal antibodies. Callum Chapman is the PQL for multiple molecules across AstraZeneca’s pipeline, working mostly with inhaled biologics and advanced therapy medicinal products. He is a UK-registered Pharmacist and is interested in the area where CMC meets clinical development.
Callum Chapman is the PQL for multiple molecules across AstraZeneca’s pipeline, working mostly with inhaled biologics and advanced therapy medicinal products. He is a UK-registered Pharmacist and is interested in the area where CMC meets clinical development. Rob Gaglione serves as a lean practitioner for AstraZeneca’s Biologics Development Quality Department. Prior to joining AstraZeneca, he worked as a supplier quality professional in the medical device industry.
Rob Gaglione serves as a lean practitioner for AstraZeneca’s Biologics Development Quality Department. Prior to joining AstraZeneca, he worked as a supplier quality professional in the medical device industry. Andy Spasoff has worked in the biotechnology industry for over 18 years at multiple large companies. He has spent time in Process Development, Global Operations and Quality focused on the commercialization and commercial support of biologic products.
Andy Spasoff has worked in the biotechnology industry for over 18 years at multiple large companies. He has spent time in Process Development, Global Operations and Quality focused on the commercialization and commercial support of biologic products. Anthony Mire-Sluis, PhD, is currently Head of Global Quality at AstraZeneca. Prior to working at AstraZeneca, he held the role of Vice President of Quality at Amgen. He was also Principal Advisor, Regulatory Science and Review, Office of Biotechnology Products, CDER and Head of Analytical Sciences and Standards, Office of the Director, CBER, U.S. FDA.
Anthony Mire-Sluis, PhD, is currently Head of Global Quality at AstraZeneca. Prior to working at AstraZeneca, he held the role of Vice President of Quality at Amgen. He was also Principal Advisor, Regulatory Science and Review, Office of Biotechnology Products, CDER and Head of Analytical Sciences and Standards, Office of the Director, CBER, U.S. FDA.