Validating Recombinant Endotoxin Reagents Why Indigenous Microorganism Testing Is Essential
Pharmaceutical manufacturers face a critical dilemma: how to increase reliability and reduce risk in endotoxin testing while simultaneously addressing pressing sustainability concerns and evolving supply chain considerations. For decades, the traditional Limulus Amebocyte Lysate (LAL) assay, derived from horseshoe crab blood, has been the gold standard for bacterial endotoxin detection to ensure product safety. Yet as the industry evolves, so do its priorities – globally, over 80 million endotoxin tests are performed annually, with the majority relying on LAL reagents. With increasing awareness of environmental stewardship and the pharmaceutical industry's commitment to animal-free methods, there is a drive to implement more sustainable alternatives. The emergence of recombinant Factor C (rFC) reagents and recombinant Cascade Reagents (rCR) offers a compelling solution that addresses both reliability and sustainability. These synthetic alternatives eliminate the need for animal-derived materials while delivering improved accuracy, reproducibility, and specificity compared to traditional LAL reagents. However, successful adoption of these recombinant technologies requires thorough validation, with particular emphasis on testing against autochthonous microorganisms. This article explores the critical importance of evaluating indigenous microorganisms when transitioning to recombinant reagents, enabling manufacturers to achieve objectives related to both risk reduction and sustainability.
The Evolution of Endotoxin Testing: From LAL to Recombinant Alternatives
Bacterial endotoxins – lipopolysaccharides (LPS) found in the outer membrane of Gram-negative bacteria – are potent pyrogens that can cause severe adverse reactions in humans, including fever, septic shock, and even death. For decades, the LAL assay has been an indispensable tool for detection, based on the clotting cascade initiated by endotoxin in the presence of Factor C, B, and proclotting enzyme.
Despite its proven efficacy, the LAL assay faces increasing scrutiny as the pharmaceutical industry shifts away from animal-derived reagents. This has accelerated the development of synthetic alternatives, including rCR and rFC. rFC reagents mimic the initial step of the LAL cascade, directly binding to and being activated by endotoxin to produce a measurable signal. rCR reagents, in contrast, mimic the entire LAL cascade. These reagents offer several advantages: a sustainable supply, reduced batch-to-batch variability, and often, enhanced specificity for endotoxins.
Understanding the differences between rFC and rCR
Recombinant Factor C (rFC) is a fluorescence-based endpoint assay that relies on a single recombinant protein clone – Factor C – to detect bacterial endotoxins. The assay is incubated for a set time period, with readings taken at the beginning and end of the test. When endotoxins are present, the fluorescence reader amplifies the fluorogenic substrate. Because rFC contains only the Factor C clone and not the full cascade, it does not detect Beta-Glucans.
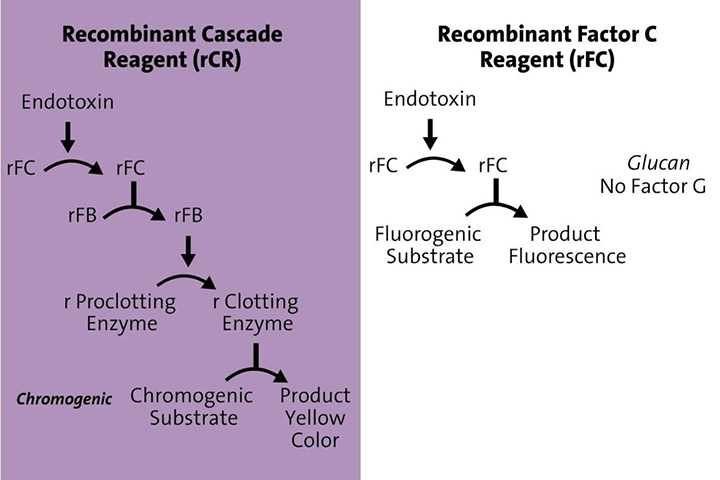
Recombinant Cascade Reagent (rCR), by contrast, is based on an absorbance, kinetic assay that more closely mimics the traditional LAL cascade. rCR uses the same reader that current LAL uses and takes many readings over time. The rCR assay utilizes recombinant Factor C, recombinant Factor B, and recombinant proclotting enzyme to replicate the natural enzymatic cascade. This kinetic chromogenic approach takes multiple readings over time to measure color change, similar to traditional kinetic chromogenic LAL methods.
Mechanisms of Potential Interference
The potential for interference from outside materials can manifest through several mechanisms:
- Non-LPS Microbial Components: Some fungi or other microorganisms produce cell wall components (e.g., peptidoglycans, β-glucans, teichoic acids) or extracellular products that could potentially interact with recombinant proteins or other components of the reagent. While rFC and rCR are designed to be highly specific for lipid A, the active component of LPS, subtle structural similarities or non-specific binding may occur under certain conditions, particularly at high concentrations of interfering substances.
- Enzymatic Activity: Certain microbial enzymes could potentially degrade or modify components of the rFC or rCR reagent, or alter the endotoxin itself, leading to reduced signal or altered reaction kinetics. Endotoxins from different microorganisms can have varying serotypes, which can result in different recoveries.
- pH or Ionic Strength Alterations: Microbial growth can alter the local pH or ionic strength of a sample. While bacterial endotoxin assays are typically buffered, significant deviations caused by microbial activity could affect enzyme activity or protein conformation within the assay system.
- Biofilm Formation: Microorganisms can form biofilms on surfaces, potentially leading to localized concentrations of microbial components that might not be fully dispersed or neutralized during sample preparation, thereby creating localized interferences.

The Indispensable Role of Autochthonous Microorganism Testing
Given these potential interference mechanisms, a robust validation strategy for adopting rFC or rCR reagents must include comprehensive testing with autochthonous microorganisms relevant to the manufacturing environment. This testing goes beyond the standard validation requirements for specificity against common Gram-negative bacteria and fungi typically performed by reagent manufacturers. Instead, it focuses on the specific microbial flora that your facility, raw materials, and processes might harbor – ensuring the recombinant assay performs reliably under your actual operating conditions.
Key Considerations for Testing
- Microorganism Selection:
 Environmental Isolates: The most critical microorganisms to test are those routinely isolated from environmental monitoring
programs (air, surfaces), water systems (purified water, water for injection), and raw materials within your specific manufacturing facility. These are the microbes most likely to be present in your product stream.
Environmental Isolates: The most critical microorganisms to test are those routinely isolated from environmental monitoring
programs (air, surfaces), water systems (purified water, water for injection), and raw materials within your specific manufacturing facility. These are the microbes most likely to be present in your product stream.- Product-Specific Isolates: If your product or manufacturing process has a history of microbial excursions, those specific isolates should be prioritized.
- Diverse Representative Species: Select a diverse range of Gram-negative bacteria (e.g., Burkholderia species, Pseudomonas species), yeasts (e.g., Candida albicans), and molds (e.g., Aspergillus niger) that represent the typical microbial landscape of your operations.
- Preparation of Microbial Suspensions:
- Stressed and Non-Stressed Cells: Test both stressed and heat-killed or inactivated microbial suspensions. Heat treatment can sometimes alter cell wall components, so evaluating both states provides a more comprehensive picture.
- Concentration Range: Test a range of microbial concentrations, including levels significantly higher than those typically encountered in routine monitoring, to challenge the robustness of the assay.
- Testing Strategy:
- Spike Recovery Studies: The primary method involves spiking known concentrations of endotoxin into samples containing high concentrations of lab-cultured microorganisms. The recovery of the endotoxin should fall within an acceptable range (e.g., 50-200%). This assesses whether the presence of the microorganism can be recovered in that sample matrix.
- Matrix Effects: Consider testing microorganisms in relevant product matrices, as the product itself can sometimes influence assay performance.
- Growth Conditions: If relevant, consider the impact of different growth conditions for the microorganisms, as this can sometimes alter their cellular composition.
- Acceptance Criteria:
- Clearly define acceptance criteria for spike recovery and direct assay results. These criteria should align with regulatory expectations for endotoxin assay validation. For example, endotoxin recovery between 50-200% in the presence of microorganisms, and no detectable endotoxin for negative controls.
Benefits of Autochthonous Microorganism Testing
- Enhanced Patient Safety: By identifying and mitigating potential interferences, autochthonous testing directly contributes to ensuring the safety of pharmaceutical and medical device products.
- Robustness of the Assay: It demonstrates that the chosen rFC or rCR assay performs reliably in the presence of the specific microbial challenges inherent to your manufacturing environment.
- Regulatory Compliance: Regulatory bodies increasingly expect a thorough understanding of potential risks and a comprehensive validation package when adopting new analytical methods. Demonstrating microorganism testing provides strong evidence of due diligence.
- Risk Mitigation: Proactive identification of interferences allows for the implementation of control measures, such as adjusting sample preparation, optimizing dilution schemes, or even re-evaluating the suitability of a particular rFC or rCR reagent.
- Confidence in Results: Ultimately, it provides greater confidence in the endotoxin test results, knowing that they are not being compromised by non-endotoxin microbial components.

Practical Considerations and Best Practices
- Collaboration: Foster close collaboration between microbiology, quality control, and process development teams to ensure a comprehensive understanding of potential microbial risks and to facilitate the collection of relevant isolates. Leverage the Primary Validation Package from the recombinant reagent manufacturer.
- Documentation: Meticulously document all aspects of the microorganism testing, including isolate selection, growth conditions, preparation of suspensions, testing protocols, raw data, and acceptance criteria.
- Continuous Monitoring: While comprehensive initial validation is crucial, it's also important to consider a strategy for re-evaluating a Contamination Control Strategy if there are significant changes to raw materials, manufacturing processes, or if new microbial excursions occur.
- Reagent Selection: The results of microorganism testing can also inform the selection of the most suitable rFC or rCR reagent for your specific application. Some rFC or rCR reagents may exhibit better tolerance to certain endotoxins and interferences than others.
Conclusion
 The transition from LAL to recombinant reagents for Bacterial Endotoxins Testing represents a significant advancement in addressing the dual imperatives
pharmaceutical manufacturers face: increasing reliability and reducing risk while advancing sustainability goals. However, this transition is not merely a plug-and-play replacement. A critical – and often underemphasized – aspect of validation
is the thorough evaluation of differences between microorganisms, while also relying on the recombinant reagent manufacturer’s Primary Validation Package. By meticulously testing with the specific microbial flora present in your manufacturing
environment, you can proactively identify and mitigate risks, ensure the robustness and accuracy of your endotoxin testing programs, and ultimately, safeguard patient health. This diligent approach to validation underscores the commitment to scientific
rigor and responsible innovation within the life sciences industry. As the pharmaceutical industry continues to evolve toward more sustainable and reliable testing methods, the validation of recombinant endotoxin reagents with indigenous microorganisms
stands as an essential step in achieving both risk reduction and sustainability objectives.
The transition from LAL to recombinant reagents for Bacterial Endotoxins Testing represents a significant advancement in addressing the dual imperatives
pharmaceutical manufacturers face: increasing reliability and reducing risk while advancing sustainability goals. However, this transition is not merely a plug-and-play replacement. A critical – and often underemphasized – aspect of validation
is the thorough evaluation of differences between microorganisms, while also relying on the recombinant reagent manufacturer’s Primary Validation Package. By meticulously testing with the specific microbial flora present in your manufacturing
environment, you can proactively identify and mitigate risks, ensure the robustness and accuracy of your endotoxin testing programs, and ultimately, safeguard patient health. This diligent approach to validation underscores the commitment to scientific
rigor and responsible innovation within the life sciences industry. As the pharmaceutical industry continues to evolve toward more sustainable and reliable testing methods, the validation of recombinant endotoxin reagents with indigenous microorganisms
stands as an essential step in achieving both risk reduction and sustainability objectives.
Learn more about recombinant testing here.



