Visual Inspection Practices of Cleaned Equipment: Part I

Photo courtesy of Steris.
Regulatory and compendial guidelines require that manufacturers confirm process equipment is visually clean following a cleaning operation. Recently, a Steris survey showed that visual inspection practices for cleaned equipment differ among manufacturers. At the same time, the survey indicated that while practices and even terminology may differ, this can be accepted by regulators provided processes are well documented.
First, a word about terminology, many regulatory guidelines and industry technical documents use different terms to describe the step confirming process equipment is visually clean after cleaning. For the purposes of this article, the authors use the term “visual inspection.” Other common terms for this step are ”visual check” or ”visual examination.” Note that in this case, “visual inspection” does not refer to visibly checking finished product for particles.
For the survey, 39 responded, representing many European pharmaceutical and biopharmaceutical companies (27 companies in 34 different sites). The manufacturers who completed the survey were: 54% non-sterile (e.g., tablet, liquid, combined product), 13% sterile (e.g., biotechnology, liquid, and lyophilized product), 26% vaccine, 5% medical device, 2% other (e.g., early clinical production).
The European Annex 15 states that “A visual check for cleaning is an important part of the acceptance criteria for cleaning validation” (1). Visual inspection is a critical step to confirm the effectiveness of cleaning process equipment after cleaning. The acceptance criteria for visual inspection is visually clean. The visual inspection should include direct and indirect product contact surfaces, and requires the equipment surfaces to be visible. When this is not the case, dismantling the equipment to gain access, or using tools such as mirrors, light sources, or borescopes may be required (2, 3). Modern technology such as digital cameras capable of assessing the surface may also be explored for large-volume vessels, when a visual inspection is challenging.
Visually clean is the minimum standard expected. An additional acceptance criterion, such as the health-based limit, however, is enforced (1-5). Therefore, a validated analytical method with a sensitivity below the cleaning limit should be periodically coupled with a visual inspection. When applying only visual inspection to determine the cleanliness of equipment, the threshold at which the product is readily visible as a residue should be established (3, 6).
The visual inspection is always performed (to whatever degree possible) at the end of a complete cleaning cycle (7). The visual inspection is an active and qualitative observation of product contact surfaces to confirm the absence of residue and the next batch production can start (7). ICH Q7: Practice Guidance for Active Pharmaceutical Ingredients states that: “12.76 … Visual inspection can allow detection of gross contamination concentrated in small areas that could otherwise go undetected by sampling and/or analysis.”
Back to the survey, 54% of respondents are performing a visual inspection of the equipment surface when it is dry (Figure 1).
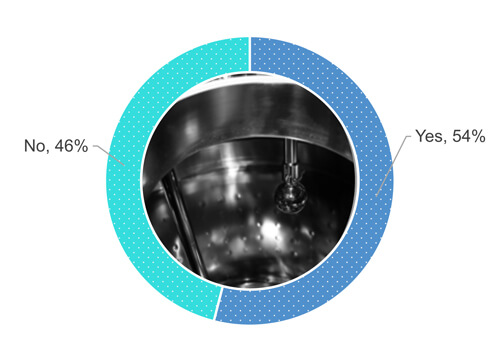 Figure 1 Does your company procedure require you to visually inspect the process equipment surface after a cleaning operation when the surface is dry?
Figure 1 Does your company procedure require you to visually inspect the process equipment surface after a cleaning operation when the surface is dry?
Some technical industry documents suggest that visual inspection should be performed on a dried surface, when possible, to avoid false-negative results (8,9):
- Active Pharmaceutical Ingredients Committee Guidance (10):
- “After cleaning procedures are performed, equipment should be dried to allow the visual inspection.”
- “The acceptance criteria for equipment cleaning should be based on visually clean in dry conditions and an analytical limit.”
- PDA Technical Report No. 29: Points to Consider for Cleaning Validation (9): “Ordinarily surfaces that are visually examined should be dry, as this represents a worst-case condition for visual inspection.”
It is commonly known that for some residues, visually clean may only be achieved when the surface is wet, while this is no longer the case when the surface dries.
Equipment design and cleaning cycle parameters may conduct to dry the equipment surfaces, such as having the piping sloped toward the drain, being self-drainable, and the final rinse performed at high temperatures. In some cases, clean air blown into the equipment and the distribution system may help to achieve dryness on surfaces (9).
Despite these parameters listed above being met, however, droplets or moisture (“sweat”) on the surface could potentially be observed and may be acceptable if adequately justified.
As Figure 1 shows, 48% out of 54% who responded “yes,” when asked if they visually inspect equipment surfaces when entirely dry (Figure 2). 24% out of the 54% allow some partial wetness (e.g., isolated drops or sweat) of the surfaces during a visual inspection of the cleaned equipment (Figure 2). 19% out of the 54% accept numerous drops but no pooling of water. 9% out of the 54% have not defined the dryness requirements.
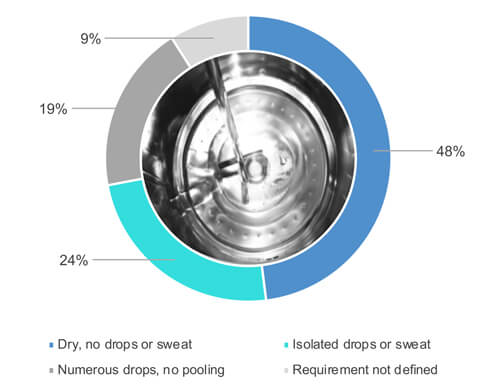 Figure 2 What is the dryness requirement for process equipment following the cleaning cycle is finished?
Figure 2 What is the dryness requirement for process equipment following the cleaning cycle is finished?
Different levels of dryness are acceptable if adequately justified and backed up by data demonstrating the absence of impact on the visual inspection post-cleaning and bioburden proliferation during clean storage, e.g., robust instruction and training using photos to avoid deviation. Finally, none of the 54% of the manufacturers that visually inspect their equipment when the surfaces are dry authorize for standing or pooling water on the equipment surfaces, as suggested in the U.S. FDA inspections guide (10): “…For example, equipment should be dried before storage, and under no circumstances should stagnant water be allowed to remain in equipment subsequent to cleaning operations.”
Considerable responsibility is given to an operator to decide whether equipment surfaces are visually clean. Therefore, the operators should be able to inspect all surfaces of the equipment visually. If not possible, adequate or advanced tools should be available to guarantee proper decision making (2, 3, 9, 11). A visual inspection of a large vessel through a sight glass is restrictive due to hidden surface. EMA suggests that the ability to visually inspect the equipment, such as distances observed in the field, should be taken into consideration in a Q&A document (3), recommending that “written instructions specifying all areas requiring visual inspection should be in place and records clearly confirm that all inspections are completed.” Finally, detailed procedures and training on visually clean criteria are mandatory to ensure correct decision making. The level of training and qualification to visually inspect should be commensurate by the risk of cross-contamination (3, 7, 9, 11).
When it comes to photos, 38% of the respondent surveyed have defined visually clean criteria by also adding photos and examples of clean status (Figure 3). 46% rely on the operator’s understanding of visually clean, coupled with a theoretical definition of visually clean in the procedure. Finally, 16% (3% and 13%) rely on the operator’s experience and training (using specific support or pictures) of a visually clean surface (Figure 3).
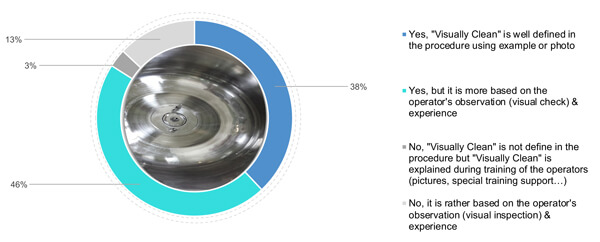 Figure 3 Does your company define and detail the meaning of “visually clean” criteria?
Figure 3 Does your company define and detail the meaning of “visually clean” criteria?
Operators performing visual inspection require specific training because what one can visually see varies with the distance, angle, lighting, the nature of the surface, the dryness level and the inspector’s visual acuity (3, 7, 9). EMA suggests that eyesight testing (or visual acuity) should be performed periodically, and the competency of the operator should be proven through a practical assessment (3). The ISPE Risk Mapp suggests that: “In situations where the method of detection is visual only, it is important to understand the visual acuity of the staff, and what level of residue is considered safe (7). If the safe level is below the visual acuity of the staff then the risk of failure not being detected may be considered high whereas if the safe level is well above (several orders of magnitude), the visual acuity of the staff the risk of failure not being detected may be considered low.”
The frequency of visual acuity test and visual acuity limit would depend on specific variable such as the:
- distance between the inspector and the inspected equipment surface (3, 7, 12)
- equipment configuration and surface nature (7,8)
- surrounding lighting conditions (3, 7, 9, 12)
- frequency of analytical testing (3, 7-9, 11,12)
- residues toxicity, cleaning limit versus visual detection limit (3,6,7)
- visual inspection only is performed to confirm the cleanliness of an equipment (7)
38% of the respondents do not require an operator’s certification or qualification (Figure 4). Yet 49% train their operators to visually clean on the field (in front of the equipment). Finally, 13 % qualify their operators.
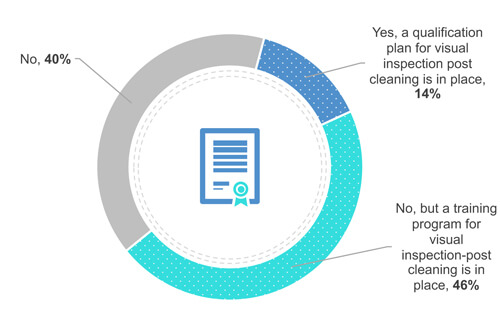 Figure 4 Does your company have a certification or qualification program in place for visual inspection?
Figure 4 Does your company have a certification or qualification program in place for visual inspection?
Some manufacturers have defined the visual residue limit, using coupons, an operator could detect (13, 14). Also, some of them mimicked the distance between the operator and the surfaces to be inspected on process equipment (15, 16). When the visual residue limit is lower than the cleaning limit visual inspection alone as an acceptance criterion may be enough (7).
Does a certification or qualification along with a training program ensure operators’ competency to inspect a surface visually? Following the precept of ICH Q9: Quality Risk Management (17), the answer would generally depend on the:
- number of historical visual inspection deviation due to operator training
- difficulty in inspecting the equipment (equipment configuration and background environment such as lighting, angle, etc.) (3, 7–9, 11, 12, 18, 19)
- type of visual inspection being used; qualitative or quantitative visual inspection (7,12, 13–16, 18,19)
- visual inspection is not supplemented with analytical testing (13–16,18)
- frequency of analytical testing performed with visual inspection
- analytical method sensibility against the cleaning limit, toxicity (based on the health-based exposure limit) of the residues (1–7, 9,11)
- presence of a double-check
Conclusion
The visual inspection concept and “visually clean” criteria seem to vary among European manufacturers based on their experience with the cleaning process execution and understanding of the regulatory requirements. The visually clean criteria, however, must be clearly defined in the procedures. Operators performing visual inspection require specific training, which can be based on their own experiences.
The variability in practices between manufacturers, as suggested by the survey results, may be acceptable when the risk is documented. Following the precepts of ICH Q9, the level of effort and formality should be commensurate with the patient’s risk.
In Part II, a case study and suggested minimal requirement for visual inspection of cleaned surfaces will be presented.
Author note: We wish to sincerely thank all those who participated in the survey.
References
- European Commission, Good Manufacturing Practice Medicinal Products for Human and Veterinary Use: Annex 15, qualification and validation, 2015.
- Canada Health Products and Food Branch Inspectorate. Guidance Document. Cleaning validation guidelines: Drug and health products. Health Canada: Ottawa, Canada; 2002 Spring.
- European Medicines Agency, Questions and answers on implementation of risk-based prevention of cross-contamination in production and ‘Guideline on setting health-based exposure limits for use in risk identification in the manufacture of different medicinal products in shared facilities’ (EMA/CHMP/CVMP/SWP/169430/2012), EMA/CHMP/CVMP/SWP/246844/2018, (April 2018)
- European GMP part IV Guidelines on Good Manufacturing Practice specific to Advanced Therapy Medicinal Products
- Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme. Guide to Good Manufacturing Practice for Medicinal Products: Annex 15, 2015.
- Walsh A. et al., “Justification & Qualification Of Visual Inspection For Cleaning Validation In A Low-Risk, Multiproduct Facility.” Pharmaceutical Online (Aug. 3, 2018) accessed January 2020 https://www.pharmaceuticalonline.com/doc/justification-qualification-of-visual-inspection-for-cleaning-validation-in-a-low-risk-multiproduct-facility-0001
- ISPE, ISPE Risk-Based Manufacture of Pharmaceutical Products, second edition, Volume 7
- Active Pharmaceutical Ingredients Committee, guidance on aspects of cleaning validation in active pharmaceutical ingredient plants (2016)
- Parenteral Drug Association, Technical Report 29, Points to Consider for Cleaning Validation (2012)
- GUIDE TO INSPECTIONS VALIDATION OF CLEANING PROCESSES, accessed on September 2019: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-guides/validation-cleaning-processes-793
- Parenteral Drug Association, Technical Report 49, Points to Consider for Biotechnology Cleaning Validation (2010)
- ASTM: E306-18, Standard guide for Science-Based and Risk-Based Cleaning Process Development and Validation, October 2018 version 1.
- Forsyth, R.J., et al. “Visible-Residue Limit for Cleaning Validation and its Potential Application in a Pharmaceutical Research Facility.” Pharmaceutical Technology 28 (Oct. 1, 2004) 68–72.
- “Application of Visible-Residue Limit for Cleaning Validation.” Pharmaceutical Technology 28 (Oct. 2, 2005) 10 http://www.pharmtech.com/application-visible-residue-limit-cleaning-valiation?id=&pageID=1&sk=&date= (accessed September 2019)
- “Determination of Surface Visible Residue Limits on Pharmaceutical Plant Equipment.” Pharmaceutical Technology 37 (Feb. 2, 2013)
- Desai, P., and Walsh, A. “Validation of Visual Inspection As An Analytical Method For Cleaning Validation.” Pharmaceutical Online (Sept. 11, 2017) https://www.pharmaceuticalonline.com/doc/validation-of-visual-inspection-as-an-analytical-method-for-cleaning-validation-0001 (accessed September 2019)
- International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for human use, Quality Risk Management Q9, (2005)
- Walsh, A., et al., “Justification & Qualification Of Visual Inspection For Cleaning Validation In A Low-Risk, Multiproduct Facility.” Pharmaceutical Online (Aug. 3, 2018) https://www.pharmaceuticalonline.com/doc/justification-qualification-of-visual-inspection-for-cleaning-validation-in-a-low-risk-multiproduct-facility-0001 (accessed January 2020)
- Forsyth, R.J., and Hartman, J., “A Risk-based Approach to Cleaning Validation using Visible Residue Limits.” Pharmaceutical Engineering 28 (2008) 8–22.



 Walid El Azab is a Technical Services Manager for the Life Sciences Division of STERIS Corporation.
Walid El Azab is a Technical Services Manager for the Life Sciences Division of STERIS Corporation.