PDA Capital Area Chapter
Chapter's Region:
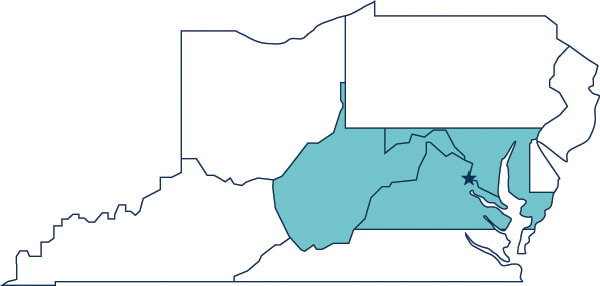
Washington, DC / Maryland / Virginia (Northern) / West Virginia
Stay Informed!
Subscribe now to receive timely updates, important announcements, and exclusive insights right in your inbox. Don't miss a moment!
Join PDA Today
Unlock exclusive resources, industry insights, and networking opportunities.
Become a MemberGet in Touch
Have questions? We're here to help! Reach out to us anytime.
Welcome to the PDA Capital Area chapter website. Here you will find information regarding all chapter activities. We encourage you to stay connected to your local industry community by participating in an upcoming chapter event or forum discussion. Chapter Leaders welcome your questions/comments and look forward to hearing from you.
Sponsors
PDA Capital Area Chapter welcomes sponsors to participate in upcoming events. We thank all the sponsors who have supported PDA Capital Area events in the past.
Please send all sponsorship inquires to Martin Jenkins, Chapter President.
Sponsorship Opportunities Book Your Sponsorship TodayFrom Vision to Vitality
The PDA Capital Area Chapter was founded in 1992 by Rande Leibowitz with guidance from PDA President Edmund Fry. From its earliest meetings and local programs, the Chapter became a cornerstone for connection between pharmaceutical professionals and regulators across the Washington, D.C. Metropolitan Area.
After nearly a decade of inactivity, the Chapter was revitalized under the leadership of Tita Tavares, whose commitment and vision re-energized local engagement. That renewal laid the groundwork for continued growth and collaboration, uniting new and returning members around shared goals of excellence and community impact which led to our Chapter being selected as 2022-2023 PDA Chapter of the Year.
Building on that foundation, the Chapter has hosted impactful programs such as Technical Reports Live and the Capital Area Roundtable. We have also continued to support the Early Career Professionals community and the Global Pharmaceutical Student (GPS) Network. Regular Membership Meetings feature expert speakers who address both technical and professional topics relevant to our members.
Today, the PDA Capital Area Chapter serves as a hub for innovation, connection, and professional development. The group advances the mission of PDA while embracing the spirit of well-being and balance.
Our philosophies:
“Bridging the Gap to the Blue Zone Lifestyle: Where Health Thrives”
“Empowering Pharma Professionals Through Networking and Innovation in the Heart of the Nation”
Together, we build bridges between science, people, and purpose, strengthening our community and the industry we serve.
Members At Large
- Sapan Patel
- Julie Barnhill
Past Presidents
- Tita Tavares, Immediate Past President and Chapter Advisor
- Allen L. Burgenson, 2005 - 2009
- Barry A. Friedman, Ph.D. 2004 - 2005
- Robert J. Mello, Ph.D. 2002 - 2004
- Allen L. Burgenson, 1999 - 2002
- William H. Stoedter, 1997- 1999
- Randy Liebowitz, 1995 - 1997
PDA Capital Area Chapter - Whiskey State of Mind Event
Get Involved
We welcome the active participation of PDA members as well as non-members as we chart our course for the future. If you would like more information about ways to get involved with the PDA Capital Area Chapter, please message our Chapter email and/or visit our Chapter LinkedIn.
Capital Area Chapter is looking for volunteers for the following committees:
- Membership, Orientation, Retention, and Enrollment (MORE) Committee
- Social Media/Communications Committee
- Program and Events Committee
- Sponsorship Committee
- Early Career Professionals / Global Pharmaceutical Student [Network] Committee
- Budget and Finance Committee
Chapter Officers
-

-
 Stephanie Brandford
Stephanie BrandfordSecretary
Brayearst Validation Consulting
Stephanie is a Carnegie Mellon chemical engineering graduate with a passion for optimization and compliance. Her career began in the chemical industry, where she focused on environmental regulations and leveraged statistical process control to enhance manufacturing processes, driving efficiency and sustainability.
Transitioning to the medical device industry, Stephanie brought these principles into the highly regulated realm of FDA standards. She dedicated herself to ensuring the safety and reliability of life-saving products, earning her Six Sigma Black Belt certification and taking on a leadership role in validation engineering. Over the past decade, she has conducted peer reviews of validation packages, identifying compliance gaps and areas for improvement. In addition, Stephanie has spent 12 years designing and delivering training programs, equipping professionals with expertise in validation and Six Sigma methodologies.
As the founder of Brayearst Validation Consulting, Stephanie leads a company dedicated to implementing Six Sigma methodologies across validation projects, training programs, and spot check services. With a deep understanding of Life Sciences products and processes, Brayearst produces robust validation documentation designed to meet stringent regulatory standards and audit requirements. Stephanie’s work reflects her unwavering commitment to precision, compliance, and advancing industry standards.
-
 Ben Bhattarai
Ben BhattaraiTreasurer
Presentations & Resources
- PDA Chapter Council Presentation 11 April 2024
- HANDOUT Final PDA Capital Area Chapter Q1 Presentation 23Apr2024
- HANDOUT PDA Capital Area Chapter Q2 Presentation 25JUN2024
- HANDOUT - PDA Capital Area Chapter Q3 Presentation 01OCT2024
- PDA Capital Area Chapter Q4 Presentation 10DEC2024
- PDA Capital Area Chapter Q1 Handout 22APR2025
- Handout_PDA Capital Area Chapter Q2 Presentation 17JUN2025
- PDA Capital Area Chapter Q3 Handout17NOV2025
- PDA Capital Area Chapter Q4 Handout 09DEC2025
Chapter Resources
Discover essential materials such as the chapter handbook, templates, presentations, and insightful videos tailored for new chapter leaders and the GPS network.
Access Resources